Marine biological shifts and climate
- PMID: 24718760
- PMCID: PMC3996605
- DOI: 10.1098/rspb.2013.3350
Marine biological shifts and climate
Abstract
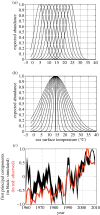
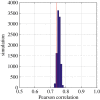
Phenological, biogeographic and community shifts are among the reported responses of marine ecosystems and their species to climate change. However, despite both the profound consequences for ecosystem functioning and services, our understanding of the root causes underlying these biological changes remains rudimentary. Here, we show that a significant proportion of the responses of species and communities to climate change are deterministic at some emergent spatio-temporal scales, enabling testable predictions and more accurate projections of future changes. We propose a theory based on the concept of the ecological niche to connect phenological, biogeographic and long-term community shifts. The theory explains approximately 70% of the phenological and biogeographic shifts of a key zooplankton Calanus finmarchicus in the North Atlantic and approximately 56% of the long-term shifts in copepods observed in the North Sea during the period 1958-2009.
Keywords: climate change; marine; shifts.
Figures


References
-
- Edwards M, Richardson AJ. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (doi:10.1038/nature02808) - DOI - PubMed
-
- Beaugrand G, Reid PC, Ibañez F, Lindley JA, Edwards M. 2002. Reorganisation of North Atlantic marine copepod biodiversity and climate. Science 296, 1692–1694 (doi:10.1126/science.1071329) - DOI - PubMed
-
- Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) - DOI - PubMed
-
- de Young B, Barange M, Beaugrand G, Harris R, Perry RI, Scheffer M. 2008. Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol. Evol. 23, 402–409 (doi:10.1016/j.tree.2008.03.008) - DOI - PubMed
-
- Beaugrand G, Edwards M, Brander K, Luczak C, Ibañez F. 2008. Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecol. Lett. 11, 1157–1168 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

