Neuraminidase inhibitors for preventing and treating influenza in adults and children
- PMID: 24718923
- PMCID: PMC6464969
- DOI: 10.1002/14651858.CD008965.pub4
Neuraminidase inhibitors for preventing and treating influenza in adults and children
Abstract
Background: Neuraminidase inhibitors (NIs) are stockpiled and recommended by public health agencies for treating and preventing seasonal and pandemic influenza. They are used clinically worldwide.
Objectives: To describe the potential benefits and harms of NIs for influenza in all age groups by reviewing all clinical study reports of published and unpublished randomised, placebo-controlled trials and regulatory comments.
Search methods: We searched trial registries, electronic databases (to 22 July 2013) and regulatory archives, and corresponded with manufacturers to identify all trials. We also requested clinical study reports. We focused on the primary data sources of manufacturers but we checked that there were no published randomised controlled trials (RCTs) from non-manufacturer sources by running electronic searches in the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE (Ovid), EMBASE, Embase.com, PubMed (not MEDLINE), the Database of Reviews of Effects, the NHS Economic Evaluation Database and the Health Economic Evaluations Database.
Selection criteria: Randomised, placebo-controlled trials on adults and children with confirmed or suspected exposure to naturally occurring influenza.
Data collection and analysis: We extracted clinical study reports and assessed risk of bias using purpose-built instruments. We analysed the effects of zanamivir and oseltamivir on time to first alleviation of symptoms, influenza outcomes, complications, hospitalisations and adverse events in the intention-to-treat (ITT) population. All trials were sponsored by the manufacturers.
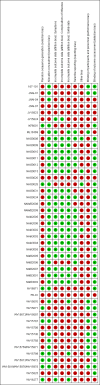
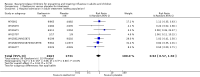
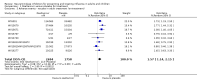
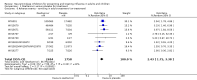
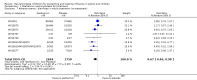
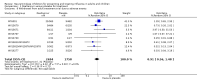
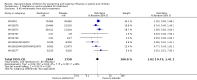
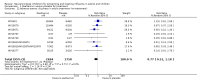
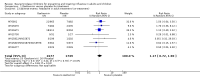
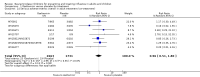
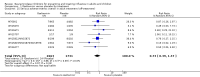
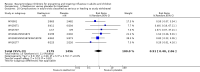
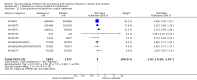
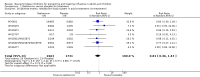
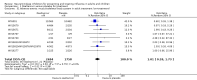
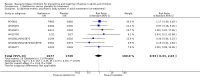
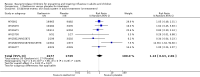
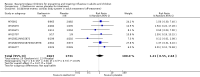
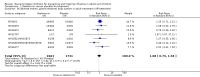
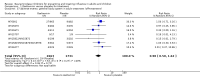
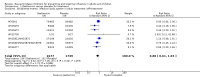
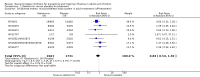
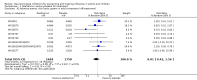
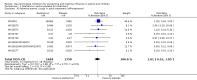
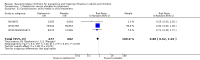
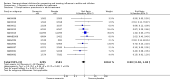
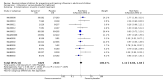
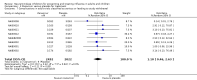
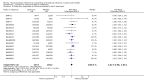
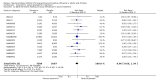
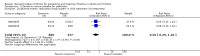
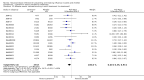
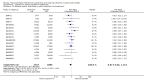
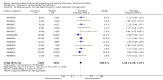
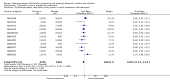
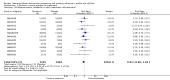
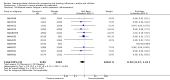
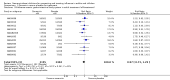
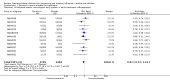
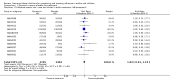
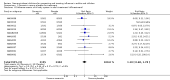
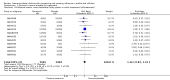
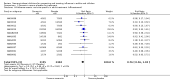
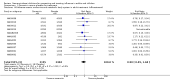
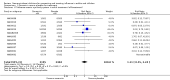
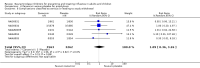
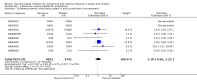
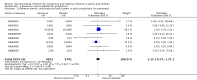
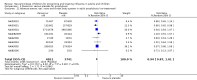
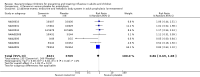
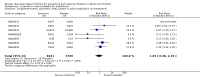
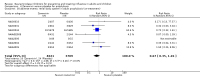
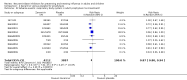
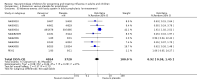
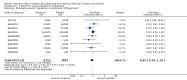
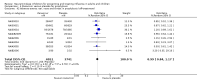
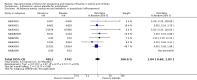
Main results: We obtained 107 clinical study reports from the European Medicines Agency (EMA), GlaxoSmithKline and Roche. We accessed comments by the US Food and Drug Administration (FDA), EMA and Japanese regulator. We included 53 trials in Stage 1 (a judgement of appropriate study design) and 46 in Stage 2 (formal analysis), including 20 oseltamivir (9623 participants) and 26 zanamivir trials (14,628 participants). Inadequate reporting put most of the zanamivir studies and half of the oseltamivir studies at a high risk of selection bias. There were inadequate measures in place to protect 11 studies of oseltamivir from performance bias due to non-identical presentation of placebo. Attrition bias was high across the oseltamivir studies and there was also evidence of selective reporting for both the zanamivir and oseltamivir studies. The placebo interventions in both sets of trials may have contained active substances. Time to first symptom alleviation. For the treatment of adults, oseltamivir reduced the time to first alleviation of symptoms by 16.8 hours (95% confidence interval (CI) 8.4 to 25.1 hours, P < 0.0001). This represents a reduction in the time to first alleviation of symptoms from 7 to 6.3 days. There was no effect in asthmatic children, but in otherwise healthy children there was (reduction by a mean difference of 29 hours, 95% CI 12 to 47 hours, P = 0.001). Zanamivir reduced the time to first alleviation of symptoms in adults by 0.60 days (95% CI 0.39 to 0.81 days, P < 0.00001), equating to a reduction in the mean duration of symptoms from 6.6 to 6.0 days. The effect in children was not significant. In subgroup analysis we found no evidence of a difference in treatment effect for zanamivir on time to first alleviation of symptoms in adults in the influenza-infected and non-influenza-infected subgroups (P = 0.53). Hospitalisations. Treatment of adults with oseltamivir had no significant effect on hospitalisations: risk difference (RD) 0.15% (95% CI -0.78 to 0.91). There was also no significant effect in children or in prophylaxis. Zanamivir hospitalisation data were unreported. Serious influenza complications or those leading to study withdrawal. In adult treatment trials, oseltamivir did not significantly reduce those complications classified as serious or those which led to study withdrawal (RD 0.07%, 95% CI -0.78 to 0.44), nor in child treatment trials; neither did zanamivir in the treatment of adults or in prophylaxis. There were insufficient events to compare this outcome for oseltamivir in prophylaxis or zanamivir in the treatment of children. Pneumonia. Oseltamivir significantly reduced self reported, investigator-mediated, unverified pneumonia (RD 1.00%, 95% CI 0.22 to 1.49); number needed to treat to benefit (NNTB) = 100 (95% CI 67 to 451) in the treated population. The effect was not significant in the five trials that used a more detailed diagnostic form for pneumonia. There were no definitions of pneumonia (or other complications) in any trial. No oseltamivir treatment studies reported effects on radiologically confirmed pneumonia. There was no significant effect on unverified pneumonia in children. There was no significant effect of zanamivir on either self reported or radiologically confirmed pneumonia. In prophylaxis, zanamivir significantly reduced the risk of self reported, investigator-mediated, unverified pneumonia in adults (RD 0.32%, 95% CI 0.09 to 0.41); NNTB = 311 (95% CI 244 to 1086), but not oseltamivir. Bronchitis, sinusitis and otitis media. Zanamivir significantly reduced the risk of bronchitis in adult treatment trials (RD 1.80%, 95% CI 0.65 to 2.80); NNTB = 56 (36 to 155), but not oseltamivir. Neither NI significantly reduced the risk of otitis media and sinusitis in both adults and children. Harms of treatment. Oseltamivir in the treatment of adults increased the risk of nausea (RD 3.66%, 95% CI 0.90 to 7.39); number needed to treat to harm (NNTH) = 28 (95% CI 14 to 112) and vomiting (RD 4.56%, 95% CI 2.39 to 7.58); NNTH = 22 (14 to 42). The proportion of participants with four-fold increases in antibody titre was significantly lower in the treated group compared to the control group (RR 0.92, 95% CI 0.86 to 0.97, I(2) statistic = 0%) (5% absolute difference between arms). Oseltamivir significantly decreased the risk of diarrhoea (RD 2.33%, 95% CI 0.14 to 3.81); NNTB = 43 (95% CI 27 to 709) and cardiac events (RD 0.68%, 95% CI 0.04 to 1.0); NNTB = 148 (101 to 2509) compared to placebo during the on-treatment period. There was a dose-response effect on psychiatric events in the two oseltamivir "pivotal" treatment trials, WV15670 and WV15671, at 150 mg (standard dose) and 300 mg daily (high dose) (P = 0.038). In the treatment of children, oseltamivir induced vomiting (RD 5.34%, 95% CI 1.75 to 10.29); NNTH = 19 (95% CI 10 to 57). There was a significantly lower proportion of children on oseltamivir with a four-fold increase in antibodies (RR 0.90, 95% CI 0.80 to 1.00, I(2) = 0%). Prophylaxis. In prophylaxis trials, oseltamivir and zanamivir reduced the risk of symptomatic influenza in individuals (oseltamivir: RD 3.05% (95% CI 1.83 to 3.88); NNTB = 33 (26 to 55); zanamivir: RD 1.98% (95% CI 0.98 to 2.54); NNTB = 51 (40 to 103)) and in households (oseltamivir: RD 13.6% (95% CI 9.52 to 15.47); NNTB = 7 (6 to 11); zanamivir: RD 14.84% (95% CI 12.18 to 16.55); NNTB = 7 (7 to 9)). There was no significant effect on asymptomatic influenza (oseltamivir: RR 1.14 (95% CI 0.39 to 3.33); zanamivir: RR 0.97 (95% CI 0.76 to 1.24)). Non-influenza, influenza-like illness could not be assessed due to data not being fully reported. In oseltamivir prophylaxis studies, psychiatric adverse events were increased in the combined on- and off-treatment periods (RD 1.06%, 95% CI 0.07 to 2.76); NNTH = 94 (95% CI 36 to 1538) in the study treatment population. Oseltamivir increased the risk of headaches whilst on treatment (RD 3.15%, 95% CI 0.88 to 5.78); NNTH = 32 (95% CI 18 to 115), renal events whilst on treatment (RD 0.67%, 95% CI -2.93 to 0.01); NNTH = 150 (NNTH 35 to NNTB > 1000) and nausea whilst on treatment (RD 4.15%, 95% CI 0.86 to 9.51); NNTH = 25 (95% CI 11 to 116).
Authors' conclusions: Oseltamivir and zanamivir have small, non-specific effects on reducing the time to alleviation of influenza symptoms in adults, but not in asthmatic children. Using either drug as prophylaxis reduces the risk of developing symptomatic influenza. Treatment trials with oseltamivir or zanamivir do not settle the question of whether the complications of influenza (such as pneumonia) are reduced, because of a lack of diagnostic definitions. The use of oseltamivir increases the risk of adverse effects, such as nausea, vomiting, psychiatric effects and renal events in adults and vomiting in children. The lower bioavailability may explain the lower toxicity of zanamivir compared to oseltamivir. The balance between benefits and harms should be considered when making decisions about use of both NIs for either the prophylaxis or treatment of influenza. The influenza virus-specific mechanism of action proposed by the producers does not fit the clinical evidence.
Conflict of interest statement
All review authors have applied for and received competitive research grants. TJ, PD, CDM, MT, RH, MJ and CH are co‐recipients of the NIHR grant to carry out this review (
Prof Jefferson receives royalties from his books published by Blackwell and Il Pensiero Scientifico Editore, Rome. Dr Jefferson is occasionally interviewed by market research companies for anonymous interviews about Phase 1 or 2 pharmaceutical products. In 2011‐2013 Dr Jefferson acted as an expert witness in a litigation case related to oseltamivir phosphate; Tamiflu [Roche] and in a labour case on influenza vaccines in healthcare workers in Canada. In 1997‐99 Dr Jefferson acted as consultant for Roche, in 2001‐2 for GSK and in 2003 for Sanofi‐Synthelabo for pleconaril (an anti‐rhinoviral which did not get approval from FDA). Dr Jefferson is a consultant for IMS Health.
Dr Doshi received EUR 1500 from the European Respiratory Society in support of his travel to the society's September 2012 annual congress in Vienna, where he gave an invited talk on oseltamivir.
Prof Del Mar was a Board member of two companies to commercialise research at Bond University, part of his responsibilities as Pro‐Vice Chancellor (Research) until 2010, receives fees for editorial and guideline developmental work and royalties from books, and is in receipt of institutional grants from NHMRC (Aus), NIHR (UK) and HTA (UK) and from a private donor (for support of the editorial base of the Cochrane ARI Group).
Dr Hama receives royalties from two books published in 2008 titled "Tamiflu: harmful as was afraid" and "In order to escape from drug‐induced encephalopathy". Dr Hama provided scientific opinions and expert testimony on 11 adverse reaction cases related to oseltamivir and gefitinib.
Dr Howick has received expenses and payments from Johns Hopkins and the American Society for Neurophysiological Monitoring as an EBM consultant. Dr Howick has received funding from the Wellcome Trust, the Medical Research Council of the UK, the Economics and Social Science Research Council of the UK and he is currently a National Institute for Health Research non‐clinical research fellow. He has received payment from the Canadian Medical Association Journal for writing a book review and receives royalties from the publication of his book from Blackwell/Wiley.
Dr Heneghan receives payment for running educational courses at the University of Oxford and University of Oxford ISIS consulting services for external teaching and training. He also receives royalties for books (Evidence Based Toolkit series by Blackwell BMJ Books).
Dr Onakpoya has no additional interests to disclose. Dr Thompson has no additional interests to disclose. Dr Jones has no additional interests to disclose. Dr Spencer has no additional interests to disclose. Dr Nunan has no additional interests to disclose. Dr Mahtani has no additional interests to disclose.
Figures

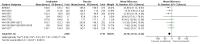

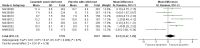


















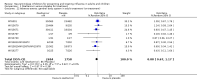
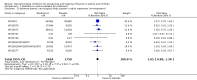
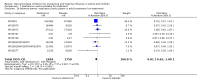




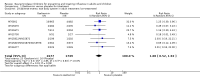

























































































































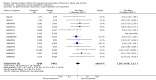
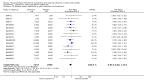
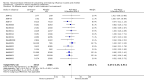
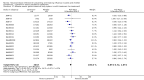



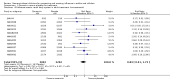
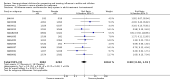

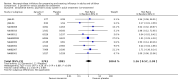

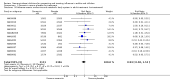










































Update of
-
Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children.Cochrane Database Syst Rev. 2012 Jan 18;1:CD008965. doi: 10.1002/14651858.CD008965.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Apr 10;(4):CD008965. doi: 10.1002/14651858.CD008965.pub4. PMID: 22258996 Updated.
Comment in
-
Cochrane review questions effectiveness of neuraminidase inhibitors.BMJ. 2014 Apr 9;348:g2675. doi: 10.1136/bmj.g2675. BMJ. 2014. PMID: 24721619 No abstract available.
-
Oseltamivir and zanamivir have limited effect on symptoms and do not reduce hospitalisation or serious complications of influenza.Evid Based Med. 2014 Dec;19(6):211. doi: 10.1136/ebmed-2014-110033. Epub 2014 Jul 1. Evid Based Med. 2014. PMID: 24985901 No abstract available.
-
Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children.Sao Paulo Med J. 2014;132(4):256-7. doi: 10.1590/1516-3180.20141324t2. Sao Paulo Med J. 2014. PMID: 25055075 Free PMC article.
-
Review: Neuraminidase inhibitors reduce symptomatic influenza; oseltamivir does not reduce hospitalizations.Ann Intern Med. 2014 Oct 21;161(8):JC2. doi: 10.7326/0003-4819-161-8-201410210-02002. Ann Intern Med. 2014. PMID: 25329219 No abstract available.
-
Neuraminidase inhibitors for treatment of influenza.Acad Emerg Med. 2021 Oct;28(10):1195-1197. doi: 10.1111/acem.14241. Epub 2021 Mar 16. Acad Emerg Med. 2021. PMID: 33635574 No abstract available.
References
References to studies included in this review
167‐101 {published data only}
-
- GSK. Investigation of the efficacy of GG167 (zanamivir) in the treatment of influenza viral infections (late Phase II study: dose comparison study). Data on file.
JNAI‐01 {published data only}
-
- GSK. Investigation of the efficacy of CG167 in the treatment of influenza viral infections (phase II study) (Protocol NoJNAI‐01). Double blind, double dummy, randomized, placebo controlled, parallel group, multicenter study to investigate safety and route of administration of CG167 when inhaled, CG167 10 mg, or the combination of inhaled CG167 10 mg plus intranasal CG167 6.4 mg, administered twice daily for 5 days in the treatment of influenza A and B viral infections. Data on file.
JNAI‐04 {published data only}
-
- GSK. A multicenter two way layout randomized placebo‐controlled double‐blind trial parallel group comparative trial on the efficacy and safety of GG167 (zanamivir) 10 mg twice a day and 20 mg twice a day in the treatment of influenza type A and type B infections (late Phase II study: dose comparison study) (Protocol No. JNAI‐04). Data on file.
JNAI‐07 {published data only}
-
- GSK. A multicenter two way layout randomized placebo‐controlled double‐blind trial parallel group comparative trial on the efficacy and safety of GG167 (zanamivir) 10 mg twice a day and 20 mg twice a day in the treatment of influenza type A and type B infections (late Phase II study: dose comparison study) (Protocol No.JNAI‐07). Data on file.
JV15823 {published data only}
-
- No authors listed. A randomized, placebo‐controlled, multicenter study of oseltamivir (Ro 64‐0796) in the treatment of influenza in Japanese subjects (Translation of summary Japanese report ‐ of 29 pages). Data on file.
JV15824 {published data only}
-
- No authors listed. Phase 3 study for prophylaxis of influenza with Ro64‐0796 (15 page summary from Japanese). Data on file.
M76001 {published data only}
-
- McGarty T. A randomized, double‐blind, placebo‐controlled, multicenter study of efficacy based on the time to treatment of influenza infection with the neuraminidase inhibitor Ro 64‐0796 (also known as GS 4104). Data on file.
ML16369 {published data only}
-
- No authors listed. A double‐blind, randomized, placebo‐controlled multicenter study of oseltamivir phosphate in the treatment of influenza infection in China. Data on file. - PubMed
NAI30008 {published data only}
-
- No authors listed. A double‐blind, randomized, placebo‐controlled, parallel group, multi‐center study to investigate the efficacy and safety of inhaled zanamivir 10 mg administered twice daily for five days in the treatment of influenza in patients 12 years or over diagnosed with asthma or chronic obstructive pulmonary disease. Data on file.
NAI30009 {published data only}
-
- Alfors S, Keene O, Grice R, Hammond J, Hendricks V, Martin N, et al. A double‐blind, randomized, placebo‐controlled, parallel‐group, multicenter study to investigate the efficacy and safety of zanamivir (GG167) 10 mg administered by inhalation twice daily for five days in the treatment of symptomatic influenza A and B viral infections in children ages 5‐12. Data on file.
NAI30010 {published data only}
-
- Hunter S, Reilly L, Sharp S, West M, Alfors S, Hammond J, et al. A double‐blind, randomized, placebo‐controlled, parallel‐group, multicenter study to investigate the efficacy and safety of inhaled zanamivir (GG167) 10 mg administered once a day for 10 days in the prevention of transmission of symptomatic influenza A and B viral infections within families. Data on file.
NAI30011 {published data only}
-
- No authors listed. A randomised, double‐blind, placebo‐controlled study to evaluate the impact of inhaled zanamivir treatment on workplace attendance due to influenza A and B infections. Data on file.
NAI30012 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled, parallel‐group, multicentre study to investigate the efficacy and safety of inhaled zanamivir 10 mg administered twice daily for five days in the treatment of symptomatic influenza A and B viral infections in subjects aged over 65 years. Data on file.
NAI30015 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled, parallel‐group, multicentre study to investigate the efficacy and safety of inhaled zanamivir 10 mg administered twice daily for five days in the treatment of symptomatic influenza A and B viral infections in armed services personnel. Data on file.
NAI30020 {unpublished data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled, multicenter study in 2 parallel groups, to investigate the efficacy and safety of inhaled zanamivir (10 mg bd. via Diskhaler), for 5 days, in high risk patients with symptomatic influenza A and / or B infection. Data on file Synopsis only available.
NAI30028 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled, multicenter study in 2 parallel groups, to investigate the efficacy and safety of inhaled zanamivir (10 mg bd via Diskhaler), for 5 days, in children aged 5 to 12 years with symptomatic influenza A and / or B infection. Data on file.
NAI30031 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled, parallel‐group, multicentre study to investigate the efficacy and safety of inhaled zanamivir 10 mg administered once a day for 10 days in the prevention of transmission of symptomatic influenza A and B viral infections within households. Data on file.
NAI30034 {published data only}
-
- No authors listed. A double‐blind, randomized, placebo‐controlled, parallel‐group, multicentre study to investigate the efficacy and safety of inhaled zanamivir 10 mg administered once a day for 28 days in the prevention of symptomatic influenza A and B viral infections in community‐dwelling high‐risk populations. Data on file.
NAIA/B2008 {published data only}
-
- No authors listed. A double‐blind, randomized, placebo‐controlled, multicenter, parallel‐group study to investigate the efficacy and safety of zanamivir administered twice or four times a day for the treatment of influenza A and B viral infections. Data on file.
NAIA/B2009 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled, multicentre, parallel‐group study to demonstrate the efficacy and safety of zanamivir in the prevention and/or progression of influenza A and B viral infections. Data on file.
NAIA2005 {published data only}
-
- MacLeod A, Gummer M, Raniga K, Hirst H, Keene O, Ossi M, et al. A double‐blind, randomised, placebo‐controlled multi‐centre study to investigate the efficacy and safety of inhaled and intranasal zanamivir in the treatment of influenza A and B viral infections. Data on file.
NAIA2006 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled multicentre study to investigate the efficacy and safety of zanamivir therapy in the prevention of progression of influenza A and B viral infections. Data on file.
NAIA3002 {published data only}
-
- Elliott M, Flack N, Keene O, Szymborski P, Vega R (PharmaResearch, Inc). A double‐blind, randomized, placebo‐controlled, parallel‐group, multicenter study to investigate the efficacy and safety of inhaled zanamivir (GG167) 10 mg administered twice a day for five days in the treatment of symptomatic influenza A and B viral infections in adolescents and adults. Data on file.
NAIA3003 {published data only}
-
- No authors listed. A double‐blind, randomized, parallel‐group, multi‐center study to investigate the efficacy and safety of inhaled zanamivir 10 mg administered once a day compared to the standard of care in controlling nursing home influenza outbreaks. Data on file.
NAIA3004 {published data only}
-
- No authors listed. A double‐blind, randomized, placebo‐controlled, parallel‐group, multi‐center study to investigate the efficacy and safety of inhaled zanamivir 10 mg once a day in controlling nursing home influenza outbreaks. Data on file.
NAIA3005 {published data only}
-
- Elliott M, Hunter S, Flack N, Crisp A, Szymborski P, Vega R. A double‐blind, randomized, placebo‐controlled, parallel‐group, multicenter study to investigate the efficacy and safety of zanamivir (GG167) 10 mg administered once a day for 28 days in the prevention of symptomatic influenza A and B viral infections in community dwelling adults. Data on file.
NAIB2005 {published data only}
-
- Leong J, Brennan J, Gummer M, Keene O, Wightman K. A double‐blind, randomised, placebo‐controlled, parallel‐group, multi‐centre study to investigate the efficacy and safety of inhaled plus intranasal zanamivir in the treatment of influenza A and B viral infections. Data on file.
NAIB2006 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled, parallel‐group, multicentre study to investigate the efficacy and safety of inhaled zanamivir in preventing progression of influenza A and B viral infections. Data on file.
NAIB2007 {published data only}
-
- Perich R, Solterbeck A, Keene O, Leong J, Raniga K, MacLeod A. A double‐blind, randomised, placebo‐controlled, parallel‐group, multi‐centre study to investigate the efficacy and safety of inhaled and inhaled plus intranasal zanamivir in the treatment of influenza A and B viral infections. Data on file.
NAIB3001 {published data only}
-
- Campion K, Gummer M, Keene O. A double‐blind, randomized, placebo‐controlled, parallel‐group, multicenter study to investigate the efficacy and safety of zanamivir administered twice daily in the treatment of influenza A and B viral infections in adults. Data on file.
NAIB3002 {published data only}
-
- Man CY, Keene ON, Challoner T (Challoner Associates). A double‐blind, randomised, placebo‐controlled, parallel‐group, multicentre study to investigate the efficacy and safety of inhaled zanamivir (GG167) 10 mg administered twice a day for five days in the treatment of symptomatic influenza A and B viral infections in adolescents and adults. Data on file.
NV16871 {published data only}
-
- No authors listed. A double‐blind, randomized, stratified, placebo‐controlled study of oseltamivir in the treatment of influenza in children with asthma. Data on file.
PE‐01 {unpublished data only}
-
- No authors listed. A trial to investigate efficacy to reduce development of influenza symptoms in influenza infected patients treated with GG167 (protocol number PE‐01 phase II clinical trial). A double‐blind double‐dummy, randomized, placebo‐controlled, parallel group, multicenter study to investigate the efficacy reducing development of influenza symptoms and, safety and rout of administration of GG 157 when orally inhaled GG 167 10 mg, 6.4 mg nebulized intranasally or the combination of inhaled GG167 10 mg plus intranasal GG167 6.4 mg was administered twice day for 5 days in the treatment of influenza A and B viral infections. Data on file.
WV15670 {published data only}
-
- Dorkings J. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomized controlled trial. Data on file.
WV15671 {published data only}
-
- Dorkings J. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. Data on file. - PubMed
WV15673/WV15697 {published data only}
-
- No authors listed. Efficacy of Ro 64‐0796 when used as chemoprophylaxis against natural influenza infection. Data on file.
WV15707 {published data only}
-
- Grosse M. A multi‐center, randomized, double‐blind, placebo‐controlled, parallel group study of oseltamivir treatment in elderly patients with influenza. Data on file.
WV15708 {published data only}
-
- No authors listed. A double‐blind randomised placebo controlled study of Ro 64‐0796 (also known as GS4104) used in elderly subjects for the prevention of clinical influenza during influenza season. Data on file.
WV15730 {published data only}
-
- Dorkings J. A double‐blind, stratified, randomized, placebo controlled study of Ro 64‐0796 (GS4104) in the treatment of influenza infection in adults. Data on file.
WV15758 {published data only}
-
- No authors listed. A double‐blind, randomized, stratified, placebo‐controlled study of Ro 64‐0796 (also known as GS 4104) in the treatment of children with influenza. Data on file.
WV15759/WV15871 {published data only}
-
- Gerster T. A double‐blind, randomized, stratified, placebo‐controlled study of oseltamivir phosphate (Ro 64‐0796, also known as GS 4104) in the treatment of influenza in children with chronic asthma. Data on file.
WV15799 {published data only}
-
- No authors listed. A double‐blind randomised placebo controlled study of Ro 64‐0796 (also known as GS4104) for the prevention of clinical influenza post exposure in families. Data on file.
WV15812/WV15872 {published data only}
-
- McCarvil M. A double‐blind, stratified, randomised, placebo controlled study of Ro 64‐0796 (also known as GS4104) in the treatment of influenza in chronically ill adults. Data on file.
WV15819/WV15876/WV15978 {published data only}
-
- No authors listed. A double‐blind, randomized, stratified, placebo‐controlled study of Ro 64‐0796 (also known as GS4104) in the treatment of influenza infection in elderly patients. Data on file.
WV15825 {published data only}
-
- No authors listed. A double‐blind, randomised, placebo‐controlled study of Ro 64‐0796 (also known as GS4104) used in elderly subjects for the prevention of clinical influenza during the influenza season. Data on file.
WV16277 {published data only}
-
- No authors listed. A double‐blind, randomised, stratified, placebo‐controlled study of oseltamivir in the treatment of influenza infection in patients. Data on file.
References to studies excluded from this review
105934 {published data only}
-
- A post‐marketing surveillance to monitor the safety of RELENZA (zanamivir) administered in Korean subjects according to the prescribing information. Data on file.
107485 {published data only}
-
- An open label, single‐dose, five‐way crossover study examining relative oral bioavailability of zanamivir with bioenhancing excipients following direct release into mid‐small intestine using gamma scintigraphy and the InteliSite Companion Capsule in healthy subjects. Data on file.
108127 {published data only}
-
- An open‐label, non‐randomized, single‐dose study to evaluate serum zanamivir pharmacokinetics following intravenous administration to human subjects with renal impairment compared to subjects without renal impairment. Data on file.
112311 {published data only}
-
- Special drug use investigation for Relenza (resistant appearance). Data on file.
112312 {published data only}
-
- Special drug use investigation for Relenza (efficacy). Data on file.
113268 {published data only}
-
- Drug use investigation for Relenza. Data on file.
113502 {published data only}
-
- Prophylactic efficacy of Relenza against influenza A and B. Data on file.
113625 {published data only}
-
- A randomized, placebo controlled, 3‐way crossover study to investigate the safety, tolerability, and pharmacokinetics of repeat dose zanamivir/placebo 10 mg administered twice daily for 5 days by a rotahaler compared to the diskhaler in healthy subjects. Data on file.
113678 {published data only}
-
- An open‐label, multi‐center, single arm study to evaluate the safety and tolerability of intravenous zanamivir in the treatment of hospitalized adult, adolescent and pediatric subjects with confirmed influenza infection. Data on file.
114045 {published data only}
-
- Collection of patients' background information Relenza® sentinel site monitoring program in Japan. Data on file.
114373 {published data only}
-
- A phase III international, randomized, double‐blind, double‐dummy study to evaluate the efficacy and safety of 300 mg or 600 mg of intravenous zanamivir twice daily compared to 75 mg of oral oseltamivir twice daily in the treatment of hospitalized adults and adolescents with influenza. Data on file.
167‐02 {published data only}
-
- A study of GG167 single blind, single administration ‐ phase I. Data on file.
167‐03 {published data only}
-
- A study of GG167 single blind, single administration ‐ phase I. Data on file.
167‐04 {published data only}
-
- A study of GG167 single blind, single administration ‐ phase I. Data on file.
167‐05 {published data only}
-
- A study of GG167 single blind, single administration ‐ phase I. Data on file.
167T3‐11 {published data only}
-
- (Zanamivir trial. Title unknown). Data on file.
ADS‐TCAD‐PO206 {published data only}
-
- A randomized open label study comparing the efficacy, safety, and tolerability of oral administration of amantadine and ribavirin with oseltamivir versus oseltamivir to influenza A virus infected immunocompromised subjects. Data on file.
BP21288 {published data only}
-
- A single‐center, open‐label, single dose, exploratory study in Caucasian and Japanese healthy subjects to investigate the pharmacokinetics of oseltamivir and its metabolite in plasma and cerebrospinal fluid. Data on file.
C94‐009 {published data only}
-
- Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clinical Pharmacokinetics. 1999;36 Suppl 1:1‐11. Not posted to GSK CTR. - PubMed
C94‐085 {published data only}
-
- Cass LM, Gunawardena KA, Macmahon MM, Bye A: Pulmonary function and airway responsiveness in mild to moderate asthmatics given repeated inhaled doses of zanamivir. Respiratory Medicine 2000;94(2):166‐73. Posted to GSK CTR. - PubMed
GCP/95/045 {published data only}
-
- A study to investigate the pharmacokinetics of GG167 in subjects with impaired renal function. Data on file.
JNAI‐02 {published data only}
-
- (Zanamivir trial. Title unknown). Data on file.
JNAI‐03 {published data only}
-
- (Zanamivir trial. Title unknown). Data on file.
JP15734 {published data only}
-
- Single ascending oral dose study of tolerability, safety and pharmacokinetics (including effect of food) of the neuraminidase inhibitor Ro 64‐0796 in healthy male volunteers. Data on file.
JP15735 {published data only}
-
- Multiple oral dose study of the tolerability, safety and PK of the neuraminidase inhibitor Ro64‐0796: direct PK comparison between Japanese and Caucasian subjects. Data on file.
JV16284 {published data only}
-
- Phase II clinical study of oseltamivir phosphate (Ro64‐0796) for the treatment of influenza in children. Data on file.
JV21490 {unpublished data only}
-
- Post‐marketing clinical study of oseltamivir phosphate on nighttime ECG in healthy adult male subjects. Data on file 2008.
M76006 {published data only}
-
- Early administration of oral oseltamivir increases the benefits of influenza treatment. Data on file. - PubMed
ML17279 {unpublished data only}
-
- An observational study to assess the accuracy of diagnosis of influenza in community pharmacies. Data on file.
ML17713 {published data only}
-
- Phase IV study on Tamiflu® capsule 75 in the elderly aged 80 years or older (a single dose oral administration study for assessing pharmacokinetics in the elderly not infected with influenza virus). Data on file.
ML19340 {unpublished data only}
-
- Impact of oseltamivir (Tamiflu ®) in post‐exposure prophylaxis influenza on mortality and morbidity in institutionalised elderly people. [Impact de l'oseltamivir (Tamiflu®) en prophylaxie antigrippale post‐exposition, sur la mortalité et la morbidité des personnes âgées institutionnalisées.]. Data on file.
ML20542 {published data only}
-
- Evaluation of combination therapy with oseltamivir and zanamivir versus monotherapy in the treatment of virologically confirmed influenza in primary care a randomised double blind controlled trial study. Data on file.
ML21954 {published data only}
-
- Efficacy and safety of combination therapies with oseltamivir & zanamivir or oseltamivir & amantadine versus oseltamivir monotherapy in the treatment of seasonal influenza A infection. Data on file.
ML22789 {published data only}
-
- An unblinded, comparative, randomized study of influenza A/H1N1 2009 resistance in patients with standard and double dose oseltamivir treatment. Data on file.
ML22872 {published data only}
-
- Viral shedding/resistance with double duration oseltamivir in infected patients (New Zealand). Data on file.
ML22879 {published data only}
-
- Viral shedding/resistance with standard dose/duration oseltamivir in infected patients (UK). Data on file.
ML25018 {published data only}
-
- A study of the relative oral bioavailability of the antiflu medicine oseltamivir (Tamiflu®) in patients in the intensive care unit. Data on file.
ML25087 {published data only}
-
- Viral shedding/resistance with double dose oseltamivir in infected patients (Australia). Data on file.
ML25094 {published data only}
-
- Nasogastric administration of OP in infected patients with respiratory failure. Data on file.
ML25157 {published data only}
-
- Oseltamivir pharmacokinetics in morbid obesity. Data on file. - PubMed
ML25176 {published data only}
-
- Open‐label pharmacokinetic of oseltamivir in healthy obese Thai adult subjects. Data on file.
ML25179 {published data only}
-
- A randomized, double‐blinded controlled trial comparing high vs standard dose oseltamivir in severe, influenza infection in ICU. "ROSII Study". Data on file.
ML25265 {published data only}
-
- Probing the functional expression of carboxyl esterase in preterm neonates using oseltamivir: a pragmatic observational study. Data on file.
ML25266 {published data only}
-
- Plasma levels of oseltamivir in H1N1 infected patients supported by extracorporeal membrane oxygenation: a single‐centre cohort study. Data on file.
MP20691 {published data only}
-
- Effect of probenecid on the pharmacokinetics of oseltamivir. Data on file.
MV20043 {published data only}
-
- A prospective study to assess household transmission of influenza and emergence and transmissibility of drug resistance to oseltamivir following treatment of children with influenza A and B. Data on file.
MV20050 {published data only}
-
- High‐dose versus standard‐dose oseltamivir for the treatment of severe influenza and avian influenza: a phase II double‐blind, randomized clinical trial. Data on file.
MV22926 {published data only}
-
- A study on higher‐dose oseltamivir treatment's impact on viral clearance and clinical recovery in adults hospitalized with influenza. Data on file. - PubMed
MV22949 {published data only}
-
- A study of the pharmacology of oseltamivir (Tamiflu) in pregnancy. Data on file.
MV22951 {published data only}
-
- Pharmacokinetics of Tamiflu® (oseltamivir) in patients receiving extracorporeal membrane oxygenation (ECMO) and or continuous venovenous hemodialysis (CVVHD). Data on file.
MV22963 {published data only}
-
- Pharmacokinetics of oseltamivir in critically ill adult patients. Data on file.
MV22970 {published data only}
-
- Observational study on the pharmacokinetics of oseltamivir in the treatment of influenza during lactation. Data on file.
NAI106784 {published data only}
-
- Phase I, open‐label study to evaluate steady‐state serum and pulmonary pharmacokinetics following intravenous administration of zanamivir in healthy adult subjects. Data on file.
NAI108166 {published data only}
NAI10901 {published data only}
-
- A double‐blind, randomised, placebo‐controlled study to evaluate the effect of inhaled zanamivir 10 mg od for 28 days on anti‐haemagglutinin antibody production (HAI titre) following co‐administration with Fluvirin™ trivalent influenza vaccine in healthy adult subjects. Data on file.
NAI10902 {published data only}
-
- An open label, randomized evaluation of the direct measurement of zanamivir concentrations in respiratory secretions following a single dose inhalation of 10 mg RELENZA™ via DISKHALER in health volunteers. Data on file.
NAI40012 {published data only}
-
- An open‐label, multi‐center study of the patient instructional leaflet for RELENZA DISKHALER. Data on file.
NAIA1009 {published data only}
-
- Pharmacokinetics of zanamivir (GG167) following inhaled administration in pediatric subjects with signs and symptoms of respiratory illness. Data on file.
NAIA2010 {published data only}
-
- Pilot, cluster randomised, open, single centre, parallel group study to evaluate the efficacy and safety of zanamivir in controlling influenza outbreaks and preventing the development of resistant influenza cases in a high risk nursing home population, compared with usual care. Data on file.
NAIB1001 {published data only}
-
- Cass LMR, Brown J, Pickford M, Fayinka S, Newman SP, Johansson CJ, et al. Pharmacoscintigraphic evaluation of lung deposition of inhaled zanamivir in healthy volunteers. Clinical Pharmacokinetics 1999 36:Suppl 1 (21‐31). Not posted to GSK CTR. - PubMed
NAIB1002 {published data only}
-
- A study to evaluate the effect of repeat doses of GG167 dry powder on pulmonary function and bronchial hyper‐responsiveness in asthmatic subjects. Data on file.
NAIB1007 {published data only}
-
- A GG167 Pharmacokinetic Study to Select a Regimen for Prophylaxis. Data on file.
NCT00297050 {published data only}
-
- A phase I double‐blind, placebo‐controlled, dose‐escalating study to evaluate the safety and tolerability of intravenous peramivir in healthy subjects. Data on file.
NCT00416962 {published data only}
-
- An open‐label, multiple dose, randomized, three‐period crossover study in healthy volunteers to evaluate the effect of co‐administration of amantadine 100 mg BID and oseltamivir 75 mg BID on the pharmacokinetic properties of amantadine and oseltamivir. Data on file.
NCT00867139 {published data only}
-
- TCAD vs. monotherapy for influenza A in immunocompromised patients. Data on file.
NCT00957996 {published data only}
-
- A phase 3, open‐label, randomized study of the antiviral activity, safety, and tolerability of intravenous peramivir in hospitalized subjects with confirmed or suspected influenza infection. Data on file.
NCT01063933 {published data only}
-
- A pharmacokinetic/pharmacodynamic and safety evaluation of investigational intravenous peramivir in children with influenza disease (CASG 117). Data on file.
Not applicable (registry) {published data only}
-
- (Oseltamivir trial. Title unknown). Data on file.
NP15525 {published data only}
-
- Multiple ascending oral dose study of the tolerability, safety and pharmacokinetics of the neuraminidase inhibitor Ro 64‐0796 in healthy volunteers. Data on file.
NP15717 {published data only}
-
- Study of the PD and PK of the neuraminidase inhibitor Ro 64‐0796 (GS4104) in the treatment of volunteers experimentally infected with human influenza B virus. Data on file.
NP15718 {published data only}
-
- An excretion balance and pharmacokinetic study of Ro 64‐0796 after a single oral dose of 14C‐labelled Ro 64‐0796 and an intravenous dose of 14C‐labelled Ro 64‐0802 in healthy male subjects. Data on file.
NP15719 {published data only}
-
- Study of the pharmacokinetics and absolute bioavailability of the neuraminidase inhibitor Ro 64‐0796. Data on file.
NP15728 {published data only}
-
- An open‐label study of the effect of cimetidine and probenecid on the pharmacokinetics of Ro 64‐0796/GS4104 in healthy subjects. Data on file.
NP15729 {published data only}
-
- An open‐label bioequivalence and food effect study of the clinical trial and market formulations of Ro 64‐0796 in healthy subjects. Data on file.
NP15743 {unpublished data only}
-
- No authors listed. A palatability study of the neuraminidase inhibitor (Ro 64‐0796), formulated as an oral formulation. Data on file.
NP15757 {published data only}
-
- No authors listed. Study of the pharmacokinetics and pharmacodynamics of the neuraminidase inhibitor Ro 64‐0796 (GS4104) in the prophylaxis of experimental infection of volunteers with the human influenza B virus. Data on file.
NP15810 {published data only}
-
- An open‐label bioequivalence and food effect study of the clinical trial and market formulations of Ro 64‐0796 in healthy subjects. Data on file.
NP15826 {published data only}
-
- An open‐label study of pharmacokinetics of Ro 64‐0796/GS4104 in children. Data on file.
NP15827 {published data only}
-
- Study of the pharmacodynamics of the neuraminidase inhibitor in the treatment of subjects experimentally infected with the human influenza B virus. Data on file.
NP15881 {published data only}
-
- Palatability testing of the neuraminidase inhibitor Ro 64‐0796 in children. Research Report No. W‐144154/27 October 1999. Data on file.
NP15901 {published data only}
-
- An open‐label, two‐way crossover pharmacokinetic drug interaction study of neuraminidase inhibitor Ro 64‐0796/GS4104 and amoxicillin in healthy volunteers. Data on file.
NP15912 {published data only}
-
- Palatability testing of the neuraminidase inhibitor Ro 64‐0796 in children. Data on file.
NP16472 {published data only}
-
- A single center, open label, multiple dose oral oseltamivir suspension study in end‐stage‐renal disease (ESRD) patients on hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD). Data on file.
NP22770 {published data only}
-
- An open‐label, multiple dose, randomized, three‐period crossover study in healthy subjects to evaluate the effect of co‐administration of oseltamivir (RO0640796) 75 mg twice daily and rimantadine 100 mg twice daily on the pharmacokinetic properties of oseltamivir and rimantadine. Data on file.
NP25138 {published data only}
-
- A study of intravenous oseltamivir [Tamiflu] in infants with influenza. Data on file.
NP25139 {published data only}
-
- A study of intravenous Tamiflu (oseltamivir) in children with influenza. Data on file.
NP25140 {published data only}
-
- PK and safety of multiple ascending doses of iv oseltamivir in healthy adults. Data on file.
NV20234 {published data only}
-
- A randomized, double‐blind trial evaluating conventional and high dose Tamiflu in the treatment of influenza in immunocompromised patients. Data on file.
NV20235 {published data only}
-
- A double‐blind, randomized, placebo controlled multicenter trial of oseltamivir for the seasonal prophylaxis of influenza in immunocompromised patients. Data on file.
NV20237 {published data only}
-
- An influenza resistance information study (IRIS). Data on file.
NV22155 {published data only}
-
- A randomized, multicenter trial of oseltamivir [Tamiflu] doses of 75 mg for 5 or 10 days versus 150 mg for 5 or 10 days to evaluate the effect on the duration of viral shedding in influenza patients with pandemic (H1N1) 2009. Data on file.
NV22158 {published data only}
-
- Avian/pandemic influenza registry final report, 30 August 2012. Data on file.
NV25118 {published data only}
-
- A randomized, multicenter, parallel study of the safety, pharmacokinetics and the effect on viral activity of intravenously administered Tamiflu [oseltamivir] in patients with influenza over 13 years of age. Data on file.
NV25182 {published data only}
-
- A prospective, observational safety study in children ≤ 24 months of age receiving oseltamivir for the treatment or prophylaxis of influenza infection. Data on file.
NV25655 {published data only}
-
- An open‐label, prospective, single oral dose study evaluating the pharmacokinetics, safety and tolerability of oseltamivir (Tamiflu) in adult subjects on peritoneal dialysis (PD) using a rapid cycle regimen to simulate automated peritoneal dialysis (APD) and in adult subjects with creatinine clearance from 10‐30 mL/min not on dialysis. Data on file.
PP15974 {published data only}
-
- A single oral dose, multi‐center, open label study of the pharmacokinetics, safety and tolerability of Ro 04‐0796/GS4104 in ESRD subjects on hemodialysis and peritoneal dialysis. Data on file.
PP16351 {published data only}
-
- An open label study of the pharmacokinetics of oseltamivir (Ro 64‐0796) in children aged 0 ‐ 5 years old after a single dose. Data on file.
PP16361 {published data only}
-
- Double‐blind, randomized, placebo controlled, single ascending i.v. dose study of the tolerability (with emphasis of nausea and vomiting), safety, pharmacokinetics of oseltamivir (Ro 64‐0796) and its active metabolite oseltamivir carboxylate (Ro 64‐0802) in healthy male volunteers. Data on file.
PV15615 {published data only}
-
- GS97802 ‐ challenge flu A treatment. Data on file.
PV15616 {published data only}
-
- GS‐97801 challenge flu A treatment. Data on file.
WP15517 {published data only}
-
- Single ascending oral dose study of the tolerability, safety and pharmacokinetics (including effect of food) of the neuraminidase inhibitor GS4104 in healthy volunteers. Data on file.
WP15525 {published data only}
-
- Multiple ascending oral dose study of the tolerability, safety and pharmacokinetics of the neuraminidase inhibitor, GS4104 in healthy volunteers. Data on file.
WP15647 {published data only}
-
- Multiple ascending oral dose study of the tolerability, safety and pharmacokinetics of the neuraminidase inhibitor Ro 64‐0796 in healthy elderly volunteers. Data on file.
WP15648 {published data only}
-
- Multiple oral dose study of the pharmacokinetics, tolerability and safety of the neuraminidase inhibitor Ro 64‐0796 in patients with renal impairment. Data on file.
WP15676 {published data only}
-
- Study of the safety and pharmacokinetics of the neuraminidase inhibitor Ro 64‐0796 in healthy volunteers when administered concomitantly with paracetamol (acetaminophen). Data on file.
WP15979 {published data only}
-
- An open‐label, relative bioavailability study of the phase III pediatric clinical trial and market formulations of Ro 64‐0796 in healthy volunteers. Data on file.
WP16094 {published data only}
-
- An open‐label, three‐way crossover, pharmacokinetic drug interaction study of neuraminidase inhibitor Ro 64‐0796 and aspirin in healthy subjects. Data on file.
WP16134 {published data only}
-
- An open label bioequivalence and food effect study of the enteric coated and immediate release formulations of oseltamivir in healthy subjects. Data on file.
WP16137 {published data only}
-
- An open‐label, bioequivalence study of the phase III pediatric clinical trial and market oral suspension formulations of Ro 64‐0796 in healthy volunteers. Data on file.
WP16225 {published data only}
-
- An open‐label, relative bioavailability study of the market suspension (with improved process), the clinical trial suspension and market capsule formulation of Ro 64‐0796 (Tamiflu, oseltamivir) in healthy subjects. Data on file.
WP16226 {published data only}
-
- A study of the pharmacokinetics of oseltamivir (Ro 64‐796) and its active metabolite Ro 64‐0802 following single oral dosing of Ro 64‐0796 to healthy volunteers and patients with moderate hepatic impairment. Data on file.
WP16254 {published data only}
-
- A pharmacokinetic drug interaction study of oseltamivir (Ro 64‐0796) and antacid in healthy volunteers. Data on file.
WP16263 {published data only}
-
- A randomized, double blind, parallel group, placebo controlled study of the effect of oseltamivir on ECG intervals in healthy subjects. Data on file.
WP16295 {unpublished data only}
-
- A randomized, open label study of the site of absorption of oseltamivir in healthy subjects using an Enterion capsule. Data on file.
WP17721 {published data only}
-
- Clinical pharmacokinetics of cyclosporine or mycophenolate with and without a concurrent single dose of oseltamivir phosphate in patients with a renal transplant. Data on file.
WP18308 {published data only}
-
- Comparison of the pharmacokinetics of Ro 64‐0802 following a single dose of oseltamivir phosphate either in a capsule or a drinking solution. Data on file.
WP20727 {published data only}
-
- A combined single ascending dose, multiple ascending dose and exploratory bioavailability study to investigate the safety, tolerability and pharmacokinetics of intravenous Ro 64‐0796 in healthy volunteers. Data on file.
WP20749 {published data only}
-
- Oseltamivir treatment for children less than 24 months of age with influenza. Data on file.
WP21272 {published data only}
-
- An open‐label, randomized 2‐period crossover study to investigate the pharmacodynamics, pharmacokinetics, safety and tolerability of warfarin, and the pharmacokinetics, safety and tolerability of oseltamivir, when given in combination. Data on file.
WP22849 {published data only}
-
- An open label, prospective, pharmacokinetic/pharmacodynamic and safety evaluation of oseltamivir (Tamiflu®) in the treatment of infants 0 to < 12 months of age with confirmed influenza infection. Data on file.
WV15731 {published data only}
-
- A double‐blind, randomized, stratified pilot study of Ro 64‐0796 (also known as GS4104) in children with influenza. Data on file.
WV16139 {published data only}
-
- (Oseltamivir trial. Title unknown). Data on file. [Mentioned in EMA EPAR dated 23 March 2006 pdf page 14 but possibly same trial as WV 16193]
WV16193 {published data only}
-
- A randomized, open‐label, parallel group study of oseltamivir used for management of influenza in households. Data on file.
References to studies awaiting assessment
JPRN‐JapicCTI‐111647 {published data only (unpublished sought but not used)}
ML20589 {published data only}
-
- Economic and social benefits of treating and preventing influenza in aged care facilities. Data on file.
ML20910 {published data only}
-
- A randomized, open label study to evaluate the effect of Tamiflu on viral shedding and on serum and cytoplasmic inflammatory cytokine concentrations in patients with laboratory‐confirmed influenza. Data on file.
ML21776 {published data only}
-
- Pilot study to develop a model to evaluate nosocomial transmission of influenza. Data on file.
MV21118 {published data only}
-
- A double‐blind, randomized, placebo‐controlled study of early oseltamivir treatment of influenza in children 1‐3 years of age. Data on file.
MV21737 {published data only}
-
- A phase 4, multi‐center, randomized, double blind, placebo controlled study, to evaluate the safety of inhaled zanamivir 10 mg versus placebo and oral oseltamivir 75 mg versus placebo for influenza prophylaxis in healthy volunteers for 16 weeks. Data on file.
MV21879 {published data only}
-
- Efficacy of oseltamivir in reducing the duration of clinical illness, viral shedding, and transmissibility reduction within households among participants in an influenza disease burden surveillance cohort in urban Dhaka, Bangladesh. Data on file.
MV22841 {published data only}
-
- Viral shedding/resistance with standard dose/duration oseltamivir in infected patients (South Africa). Data on file.
MV22940 {published data only}
-
- A randomised controlled trial on the effect of post‐exposure oseltamivir prophylaxis on influenza transmission in nursing homes (PEPpIE). Data on file.
NCT00419263 {published data only}
-
- A phase II, multicenter, randomized, double‐mask, placebo‐controlled study to evaluate the efficacy and safety of intramuscular peramivir in subjects with uncomplicated acute influenza. Data on file.
NCT00453999 {published data only}
-
- A phase II, multicenter, randomized, double‐mask, double‐dummy study comparing the efficacy and safety of peramivir administered intravenously once daily versus oseltamivir administered orally twice daily in adults with acute serious or potentially life‐threatening influenza. Data on file.
NCT00486980 {published data only}
-
- A phase 3 multicenter, randomized, double blind, placebo‐controlled study to evaluate the efficacy and safety of intramuscular peramivir in subjects with uncomplicated acute influenza. Data on file.
NCT00555893 {published data only}
-
- Monitoring influenza severity and transmission on Tamiflu (MISTT). Data on file.
NCT00610935 {published data only}
-
- A phase 3 multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of intramuscular peramivir in subjects with uncomplicated acute influenza. Data on file.
NCT00705406 {published data only}
-
- A phase II, multicenter, randomized, placebo‐controlled, study to evaluate the efficacy and safety of intramuscular peramivir 600 mg in subjects with uncomplicated acute influenza. Data on file.
NCT00958776 {published data only}
-
- A phase 3, multicenter, randomized, double‐blind, controlled study to evaluate the efficacy and safety of peramivir administered intravenously in addition to standard of care compared to standard of care alone in adults and adolescents who are hospitalized due to serious influenza. Data on file.
NCT00980109 {published data only (unpublished sought but not used)}
-
- Anekthananon T, Pukritayakamee S, Ratanasuwan W, Jittamala P, Werarak P, Charunwatthana P, et al. Oseltamivir and inhaled zanamivir as influenza prophylaxis in Thai health workers: A randomized, double‐blind, placebo‐controlled safety trial over 16 weeks. Journal of Antimicrobial Chemotherapy 2013;68:697‐707. [NCT00980109 ] - PMC - PubMed
NCT01032837 {published data only (unpublished sought but not used)}
NV20236 {published data only}
-
- An open label, multicenter trial of oseltamivir prophylaxis of seasonal influenza in children. Data on file.
Additional references
Aronson 2003
Bhatia 2007
Bourgeois 2011
-
- Bourgeois FT, Murthy S, Mandl KD. Outcome reporting among drug trials registered in ClinicalTrials.gov. Annals of Internal Medicine 2010;153:158‐66. - PMC - PubMed
Brown 2011
-
- Brown JKM. Experimental design generator and randomiser. http://www.edgarweb.org.uk/ 2011 (accessed 24 August 2011).
Burch 2009
-
- Burch J, Corbett M, Stock C, Nicholson K, Elliot AJ, Duffy S, et al. Prescription of anti‐influenza drugs for healthy adults: a systematic review and meta‐analysis. Lancet 2009;9(9):537‐45. - PubMed
Call 2005
-
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza?. JAMA 2005;8:987‐97. - PubMed
Cass 1999
-
- Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clinical Pharmacokinetics 1999;36(Suppl 1):1‐11. - PubMed
CDC 2009
-
- U.S. Centers for Disease Control and Prevention. Serum cross‐reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. Morbidity and Mortality Weekly Report 2009;58(19):521‐4. - PubMed
CDC 2013
Chou 2005
-
- Chou R, Helfand M. Challenges in systematic reviews that assess treatment harms. Annals of Internal Medicine 2005;142(2 Pt 2):1090‐9. - PubMed
Cochrane Neuraminidase Inhibitors Review Team 2011
Cohen 2009
-
- Cohen D. Complications: tracking down the data on oseltamivir. BMJ 2009;339:b5387. - PubMed
Cooper 2003
Cox 2001
-
- Cox RJ, Mykkeltvedt E, Sjursen H, Haaheim LR. The effect of zanamivir treatment on the early immune response to influenza vaccination. Vaccine 2001;19(32):4743‐9. - PubMed
Doshi 2009
-
- Doshi P. Neuraminidase inhibitors ‐ the story behind the Cochrane review. BMJ 2009;339:b5164. - PubMed
Doshi 2012a
Doshi 2012b
Doshi 2013
Dutkowski 2010
-
- Dutkowski R, Smith JR, Davies BE. Safety and pharmacokinetics of oseltamivir at standard and high dosages. International Journal of Antimicrobial Agents 2010;35:461–7. - PubMed
Ebell 2012
EMA 2010
-
- European Medicines Agency. Annex I. Summary of product characteristics (Tamiflu 30 mg hard capsule). http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?w... (accessed 21 January 2010).
EMEA 2001
-
- European Agency for the Evaluation of Medicinal Products. Tamiflu. Oseltamivir phosphate. (EMEA/H/C/402) CPMP recommendation & scientific discussion consolidated list of questions (June 28, 2001). http://www.ema.europa.eu/.
Eyding 2010
Falagas 2010
-
- Falagas ME, Koletsi PK, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, Rello J. Effectiveness and safety of neuraminidase inhibitors in reducing influenza complications: a meta‐analysis of randomized controlled trials. Journal of Antimicrobial Chemotherapy 2010;65:1330‐46. [DOI: 10.1093/jac/dkq158] - DOI - PubMed
FDA 1999a
-
- Food, Drug Administration. Administrative/correspondence documents part 2. Relenza (Zanamivir). Application No.: 21036. http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/021036‐admin2.pdf 2009 (accessed 26 August 2009).
FDA 1999b
-
- Food, Drug Administration. Medical review part 6. Relenza (zanamivir). Application No.: 21036. http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/021036‐medreview6.pdf 2009 (accessed 26 August 2009).
FDA 1999c
-
- Food, Drug Administration. Tamiflu (Oseltamivir Phosphate) Capsule. Medical Review Part 2 (Application No.: 021087). http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21087_Tamiflu_medr_... 1999 (accessed 26 August 2009).
FDA 2000a
-
- Food, Drug Administration. Letter from FDA to Hoffman‐La Roche Inc. re "NDA 21‐087 TAMIFLU (oseltamivir phosphate) MACMIS ID#8675. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati... 2000 (accessed 14 April 2000).
FDA 2000b
-
- Food, Drug Administration. Drug approval package. Relenza (zanamivir). Application No.: 021036S001. Label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/21036S1LBL.PDF 2000 (accessed 26 August 2009).
FDA 2000c
-
- Food, Drug Administration. Drug approval package. Tamiflu (oseltamivir). Application No.: 021087‐SE1‐002. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21‐087SE1‐002_rev... 2000 (accessed 27 August 2009).
FDA 2000d
-
- Food, Drug Administration. Review. Tamiflu (oseltamivir). NDA 021087 Supplement 002. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21‐087SE1‐002_rev... 2000 (accessed 26 August 2009).
FDA 2000e
-
- Food, Drug Administration. Site inspection report in Review. Tamiflu (oseltamivir). NDA 021087 Supplement 002. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21‐087SE1‐002_rev... 2000 (accessed 26 August 2009):177.
FDA 2000f
-
- Food, Drug Administration. Faxed letter to Roche (file UCM166329) [NDA 21‐087TAMIFLU (oseltamivir phosphate) MACMIS ID#8675]. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati... 2000 (accessed 19 October 2010).
FDA 2011a
-
- F. Hoffman‐La Roche. Tamiflu label (for FDA NDA no. 021087). http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021087s057lbl.pdf 2011 (accessed 7 February 2011).
FDA 2011b
-
- Food, Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/ 2011 (accessed 5 July 2011).
FDA 2011c
-
- Food, Drug Administration. Warning letters. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/default.htm 2011 (accessed 5 July 2011).
Feinberg 2009
-
- Feinberg J. Wordle. http://www.wordle.net/ 2009 (accessed 15 September 2010).
Freichel 2009
-
- Freichel C, Prinssen E, Hoffmann G, Gand L, Beck M, Weiser T, et al. Oseltamivir is devoid of specific behavioral and other central nervous system effects in juvenile rats at supratherapeutic oral doses. International Journal of Virology 2009;5(3):119‐30.
Fritz 1999
-
- Fritz RS, Hayden FG, Calfee DP, Cass LM, Peng AW, Alvord WG, et al. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo‐controlled trial of intravenous zanamivir treatment. Journal of Infectious Diseases 1999;180(3):586‐93. - PubMed
Fuiita 2011
-
- Fujita T, Fujii Y, Watanabe Y, Mori M, Yokota S. A pharmacoepidemiological study on the relationship between neuropsychiatric symptoms and therapeutic drugs after influenza infection. Japanese Journal of Pharmacoepidemiology 2010;15:73‐92.
Ghersi 2010
-
- Ghersi D, Clarke MJ, Reveiz L. Do Cochrane reviews search databases of ongoing trials, and how well do they report these searches? Oral presentation at the Joint Cochrane and Campbell Collaboration. http://www.cochrane.org/sites/default/files/uploads/abstract_book_keysto.... 2010, issue Suppl CD000002:45‐6. [DOI: 10.1002/14651858] - DOI
Godlee 2009
-
- Godlee F, Clarke M. Why don't we have all the evidence on oseltamivir?. BMJ 2009;339:b5351. - PubMed
Godlee 2010
-
- Godlee F, Loder E. Missing clinical trial data: setting the record straight. BMJ 2010; Vol. 341:c5641. [PUBMED: 20940217] - PubMed
Gotzsche 2011
-
- Gotzsche PC, Jorgensen AW. Opening up data at the European Medicines Agency. BMJ 2011; Vol. 342:d2686. [PUBMED: 21558364] - PubMed
Gravenstein 2013
-
- Gravenstein S, Peters P. Erratum. Journal of the American Geriatrics Society 2013;61:478.
Hama 2008
-
- Hama R. Fatal neuropsychiatric adverse reactions to oseltamivir: case series and overview of causal relationships. International Journal of Risk and Safety in Medicine 2008;20:5‐36.
Hama 2010
-
- Hama R, Jones M, Hayashi K, Yanagi K, Sakaguchi K. Oseltamivir: a systematic review and meta‐analysis of adverse effects in prospective cohort studies. Presentation at the 16th Japanese Society for Pharmaco‐epidemiology (JSPE) and 5th Activities and Co‐operation for Drug Safety in Asia (ACPE) Joint Meeting. Tokyo, 2010.
Hama 2011
-
- Hama R. Jones M, Hayashi K, Sakaguchi K. Oseltamivir and early deterioration leading to death. International Journal of Risk & Safety in Medicine 2011;23:201‐15. [http://iospress.metapress.com/content/5257410g24403m68/fulltext.pdf] - PubMed
Hayden 1999
-
- Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA 1999;282(13):1240‐6. - PubMed
Hayden 2009
-
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold?. Clinical Infectious Diseases 2009;48(Suppl 1):3‐13. - PubMed
Hernan 2011
HHS 2005
-
- U.S. Department of Health and Human Services. HHS pandemic influenza plan. http://www.hhs.gov/pandemicflu/plan/pdf/HHSPandemicInfluenzaPlan.pdf 2005 (accessed 9 June 2009).
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: http://www.cochrane‐handbook.org.
Huic 2011
ICH 2011
-
- The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). M4: the common technical document. http://www.ich.org/products/ctd.html 2011 (accessed 13 July 2011).
Ioannidis 2010
-
- Ioannidis JPA, Karassa FB. The need to consider the wider agenda in systematic reviews and meta‐analyses: breadth, timing, and depth of the evidence. BMJ 2010;341:c4875. - PubMed
Itoh 2009
Jack 2009
-
- Jack A. Roche steps up production of Tamiflu after virus scare. Financial Times 13 May 2009:19.
JAID 2010
-
- Japanese Association for Infectious Diseases. Question and answers on the Japanese Association for Infectious Diseases urgent recommendation "Handling pandemic influenza in routine care institutions". http://www.kansensho.or.jp/topics/090525influenza_qanda.html 2010 (accessed 9 May 2010).
Jefferson 2006
Jefferson 2009a
Jefferson 2010a
Jefferson 2011a
Jefferson 2011b
-
- Jefferson T, Doshi P, Thompson M, Heneghan C. Ensuring safe and effective drugs: who can do what it takes?. BMJ 2011;342:c7258. [PUBMED: 21224325] - PubMed
Jones 2012
-
- Jones M, Hama R, Jefferson T, Doshi P. Neuropsychiatric adverse events and oseltamivir for prophylaxis. Drug Safety 2012;35(12):1187‐8. - PubMed
Kaiser 2003
-
- Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza‐related lower respiratory tract complications and hospitalizations. Archives of Internal Medicine 2003;163:1667‐72. - PubMed
Kimura 2012
-
- Kimura S, Niwa Y, Iwajima Y, Nagano Y, Yamamoto S, Ohi Y, et al. High doses of oseltamivir phosphate induce acute respiratory arrest in anaesthetized rats. Basic & Clinical Pharmacology & toxicology 2012;111(4):232‐9. - PubMed
Lexchin 2003
Li 2007
-
- Li C, Yu Q, Ye Z, Sun Y, He Q, Li X, et al. A nonsynonymous SNP in human cytosolic sialidase in a small Asian population results in reduced enzyme activity: potential link with severe adverse reactions to oseltamivir. Cell Research 2007;17(4):357‐62. - PubMed
Liu 1995
Longini 2004
-
- Longini IM Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiology 2004;159(7):623‐33. - PubMed
Lundh 2012
MacLean 2003
-
- MacLean CH, Morton SC, Ofman JJ, Roth EA, Shekelle PG. How useful are unpublished data from the Food and Drug Administration in meta‐analysis?. Journal of Clinical Epidemiology 2003;56(1):44‐51. - PubMed
Matheson 2007
Matrosovich 2004
Maugh 2009
-
- Maugh TH II. British Medical Journal questions efficacy of Tamiflu for swine flu ‐ or any flu. http://latimesblogs.latimes.com/booster_shots/2009/12/british‐medical‐jo... 2009 (accessed 8 December 2009).
McGauran 2010
Mendel 1998
Moore 2007
-
- Moore ML, Chi MH, Zhou W, Goleniewska K, O'Neal JF, Higginbotham JN, et al. Cutting edge: oseltamivir decreases T cell GM1 expression and inhibits clearance of respiratory syncytial virus: potential role of endogenous sialidase in antiviral immunity. Journal of Immunology 2007;178(5):2651‐4. - PubMed
Moscona 2005
-
- Moscona A. Neuraminidase inhibitors for influenza. New England Journal of Medicine 2005;353(13):1363‐73. - PubMed
Nebehay 2009
-
- Nebehay S. WHO backs findings on Tamiflu for seasonal flu. http://www.reuters.com/article/idUSGEE5BA0UY20091211?type=marketsNews 2009 (accessed 11 December 2009).
NHS 2009
-
- National Health Service. NHS choices. Antivirals and swine flu. http://www.nhs.uk/news/2009/12December/Pages/Antivirals‐and‐swine‐flu.aspx 2009 (accessed 17 May 2010).
NHS 2010
-
- National Health Service. NHS Choices. Swine flu ‐ questions and answers. http://www.nhs.uk/Conditions/Pandemic‐flu/Pages/QA.aspx 2010 (accessed 17 May 2010).
NICE 2000
-
- Roche. Tamiflu (oseltamivir phosphate) NICE submission. (leaked document) 1 May 2000.
Nicholson 2000
-
- Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000;355(9218):1845‐50. - PubMed
Ohuchi 2006
-
- Ohuchi M, Asaoka N, Sakai T, Ohuchi R. Roles of neuraminidase in the initial stage of influenza virus infection. Microbes and Infection 2006;8(5):1287‐93. - PubMed
Ono 2008
-
- Ono H, Nagano Y, Matsunami N, Sugiyama S, Yamamoto S, Tanabe M. Oseltamivir, an anti‐influenza virus drug, produces hypothermia in mice. Biological and Pharmaceutical Bulletin 2008;31(4):638‐42. - PubMed
Ono 2013
-
- Ono H, Iwajima Y, Nagano Y, Chazono K, Maeda Y, Ohsawa M, et al. Reduction in sympathetic nerve activity as a possible mechanism for the hypothermic effect of oseltamivir, an anti‐influenza virus drug, in normal mice. Basic & Clinical Pharmacology & Toxicology 2013;113(1):25‐30. - PubMed
Patrozou 2009
Peters 2001
-
- Peters PH Jr, Gravenstein S, Norwood P, Bock V, Couter A, Gibbens M, et al. Long‐term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. Journal of the American Geriatrics Society 2001;49(8):1025‐31. [PUBMED: 11555062] - PubMed
Roche Investigators' Guide
-
- Roche. Investigators Guide. http://www.roche.be/fmfiles/re7189007/CU056/10_Investigators_brochure.pdf.
Rodgers 2013
Sawabuchi 2009
-
- Sawabuchi T, Suzuki S, Iwase K, Ito C, Mizuno D, Togari H, et al. Boost of mucosal secretory immunoglobulin A response by clarithromycin in paediatric influenza. Respirology 2009;14(8):1173‐9. - PubMed
Senn 2004
-
- Senn SJ. Added values: controversies concerning randomization and additivity in clinical trials. Statistics in Medicine 2004;23:3729‐53. - PubMed
Shun‐Shin 2009
Smith 2006
-
- Smith J, Dutkowski R, Ward P. Antivirals for influenza in healthy adults. Lancet 2006;367(9522):1571; author reply 1573. [PUBMED: 16698402] - PubMed
Sugaya 2010
Takahashi 2010
-
- Takahashi E, Kataoka K, Fujii K, Chida J, Mizuno D, Fukui M, et al. Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus. Microbes and Infection / Institut Pasteur 2010;12(10):778‐83. [PUBMED: 20452454] - PubMed
Tappenden 2009
-
- Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al. Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation. Health Technology Assessment 2009;11(iii, ix‐xii):1‐246. - PubMed
Toovey 2008
-
- Toovey S, Rayner C, Prinssen E. Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir. A comprehensive review. Drug Safety 2008;31(12):1097‐114. - PubMed
Toovey 2012
-
- Toovey S. The author's reply. Drug Safety 2012;35(12):1188‐90.
Treanor 2000
-
- Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000;283(8):1016‐24. - PubMed
Turner 2003
-
- Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technology Assessment 2003;7(35):iii‐iv, xi‐xiii, 1‐170. - PubMed
Van Driel 2009
-
- Driel ML, Sutter A, Maeseneer J, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. Journal of Clinical Epidemiology 2009;62:838‐44. - PubMed
Vedula 2009
-
- Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry‐sponsored trials of gabapentin for off‐label use. New England Journal of Medicine 2009;361(20):1963–71. - PubMed
Vedula 2013
Ward 2005
-
- Ward P, Small I, Smith J, Suter P, Dutkowski R. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. Journal of Antimicrobial Chemotherapy 2005;55(Suppl 1):5‐21. [PUBMED: 15709056] - PubMed
WHO 2002a
-
- World Health Organization. Global agenda on influenza‐‐adopted version. Part I. Releve Epidemiologique Hebdomadaire/Section d'Hygiene du Secretariat de la Societé des Nations = Weekly Epidemiological Record/Health Section of the Secretariat of the League of Nations 2002;77(22):179‐82. [PUBMED: 12061030] - PubMed
WHO 2002b
-
- World Health Organization. Adoption of global agenda on influenza ‐ Part II. Releve Epidemiologique Hebdomadaire/Section d'Hygiene du Secretariat de la Societé des Nations = Weekly Epidemiological Record/Health Section of the Secretariat of the League of Nations 2002;77(23):191‐5. [PUBMED: 12073536] - PubMed
WHO 2004
-
- World Health Organization. WHO guidelines on the use of vaccines and antivirals during influenza pandemics. http://www.who.int/csr/resources/publications/influenza/11_29_01_A.pdf 2009 (accessed 30 November 2009).
WHO 2007
-
- World Health Organization. WHO interim protocol: rapid operations to contain the initial emergence of pandemic influenza. http://www.who.int/influenza/resources/documents/RapidContProtOct15.pdf October 2007 (accessed 17 May 2011).
WHO 2011
-
- World Health Organization. UNEDITED REPORT of the 18th Expert Committee on the selection and use of essential medicines. http://www.who.int/entity/selection_medicines/Complete_UNEDITED_TRS_18th... 2011 (accessed 7 September 2011).
WHO 2013a
-
- World Health Organization. WHO Model List of Essential Medicines. ADULTS, 18th edition (April 2013). Rev. Oct 2013. http://www.who.int/medicines/publications/essentialmedicines/18th_EML_Fi... 2013 (accessed 25 November 2013).
WHO 2013b
-
- World Health Organization. WHO Model List of Essential Medicines for Children. 4th list (April 2013). Rev. Oct 2013. http://apps.who.int/iris/bitstream/10665/93143/1/EMLc_4_eng.pdf 2013 (accessed 25 November 2013).
Wieseler 2013
Yorifuji 2009
-
- Yorifuji T, Suzuki E, Tsuda T. Oseltamivir and abnormal behaviors: true or not?. Epidemiology 2009;20(4):619‐21. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

