miR-8 controls synapse structure by repression of the actin regulator enabled
- PMID: 24718988
- PMCID: PMC3994775
- DOI: 10.1242/dev.105791
miR-8 controls synapse structure by repression of the actin regulator enabled
Abstract
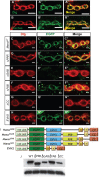
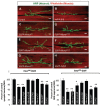
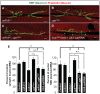
MicroRNAs (miRNAs) are post-transcriptional regulators of gene expression that play important roles in nervous system development and physiology. However, our understanding of the strategies by which miRNAs control synapse development is limited. We find that the highly conserved miRNA miR-8 regulates the morphology of presynaptic arbors at the Drosophila neuromuscular junction (NMJ) through a postsynaptic mechanism. Developmental analysis shows that miR-8 is required for presynaptic expansion that occurs in response to larval growth of the postsynaptic muscle targets. With an in vivo sensor, we confirm our hypothesis that the founding member of the conserved Ena/VASP (Enabled/Vasodilator Activated Protein) family is regulated by miR-8 through a conserved site in the Ena 3' untranslated region (UTR). Synaptic marker analysis and localization studies suggest that Ena functions within the subsynaptic reticulum (SSR) surrounding presynaptic terminals. Transgenic lines that express forms of a conserved mammalian Ena ortholog further suggest that this localization and function of postsynaptic Ena/VASP family protein is dependent on conserved C-terminal domains known to mediate actin binding and assembly while antagonizing actin-capping proteins. Ultrastructural analysis demonstrates that miR-8 is required for SSR morphogenesis. As predicted by our model, we find that Ena is both sufficient and necessary to account for miR-8-mediated regulation of SSR architecture, consistent with its localization in this compartment. Finally, electrophysiological analysis shows that miR-8 is important for spontaneous neurotransmitter release frequency and quantal content. However, unlike the structural phenotypes, increased expression of Ena fails to mimic the functional defects observed in miR-8-null animals. Together, these findings suggest that miR-8 limits the expansion of presynaptic terminals during larval synapse development through regulation of postsynaptic actin assembly that is independent of changes in synapse physiology.
Keywords: Drosophila; Enabled; NMJ; SSR; Synapse; miRNA.
Figures





Similar articles
-
Drosophila enabled promotes synapse morphogenesis and regulates active zone form and function.Neural Dev. 2020 Mar 17;15(1):4. doi: 10.1186/s13064-020-00141-x. Neural Dev. 2020. PMID: 32183907 Free PMC article.
-
Postsynaptic actin regulates active zone spacing and glutamate receptor apposition at the Drosophila neuromuscular junction.Mol Cell Neurosci. 2014 Jul;61:241-54. doi: 10.1016/j.mcn.2014.07.005. Epub 2014 Jul 24. Mol Cell Neurosci. 2014. PMID: 25066865 Free PMC article.
-
The conserved microRNA miR-34 regulates synaptogenesis via coordination of distinct mechanisms in presynaptic and postsynaptic cells.Nat Commun. 2020 Feb 27;11(1):1092. doi: 10.1038/s41467-020-14761-8. Nat Commun. 2020. PMID: 32107390 Free PMC article.
-
Embryonic and larval neural connectivity: progressive changes in synapse form and function at the neuromuscular junction mediated by cytoskeletal regulation.Wiley Interdiscip Rev Dev Biol. 2013 Nov-Dec;2(6):747-65. doi: 10.1002/wdev.114. Epub 2013 Mar 15. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24123935 Review.
-
Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration.Annu Rev Cell Dev Biol. 2003;19:541-64. doi: 10.1146/annurev.cellbio.19.050103.103356. Annu Rev Cell Dev Biol. 2003. PMID: 14570581 Review.
Cited by
-
MicroRNAs Regulate Multiple Aspects of Locomotor Behavior in Drosophila.G3 (Bethesda). 2020 Jan 7;10(1):43-55. doi: 10.1534/g3.119.400793. G3 (Bethesda). 2020. PMID: 31694853 Free PMC article.
-
MotomiRs: miRNAs in Motor Neuron Function and Disease.Front Mol Neurosci. 2017 May 4;10:127. doi: 10.3389/fnmol.2017.00127. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28522960 Free PMC article. Review.
-
The Strip-Hippo Pathway Regulates Synaptic Terminal Formation by Modulating Actin Organization at the Drosophila Neuromuscular Synapses.Cell Rep. 2016 Aug 30;16(9):2289-97. doi: 10.1016/j.celrep.2016.07.066. Epub 2016 Aug 18. Cell Rep. 2016. PMID: 27545887 Free PMC article.
-
Drosophila primary microRNA-8 encodes a microRNA-encoded peptide acting in parallel of miR-8.Genome Biol. 2021 Apr 23;22(1):118. doi: 10.1186/s13059-021-02345-8. Genome Biol. 2021. PMID: 33892772 Free PMC article.
-
MicroRNA Tissue Atlas of the Malaria Mosquito Anopheles gambiae.G3 (Bethesda). 2018 Jan 4;8(1):185-193. doi: 10.1534/g3.117.300170. G3 (Bethesda). 2018. PMID: 29146584 Free PMC article.
References
-
- Ahern-Djamali S. M., Comer A. R., Bachmann C., Kasternmeier A. S., Reddy S. K., Beckerle M. C., Wlater U., Hoffmann F. M. (1998). Mutations in Drosophila enabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. Mol. Biol. Cell 9, 2157-2171 10.1091/mbc.9.8.2157 - DOI - PMC - PubMed
-
- Bao H., Daniels R. W., MacLeod G. T., Charlton M. P., Atwood H. L., Zhang B. (2005). AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J. Neurophysiol. 94, 1888-1903 10.1152/jn.00080.2005 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

