Differential stability of PNS and CNS nodal complexes when neuronal neurofascin is lost
- PMID: 24719087
- PMCID: PMC3983793
- DOI: 10.1523/JNEUROSCI.4662-13.2014
Differential stability of PNS and CNS nodal complexes when neuronal neurofascin is lost
Abstract
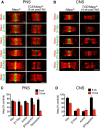
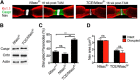
Fast, saltatory conduction in myelinated nerves requires the clustering of voltage-gated sodium channels (Nav) at nodes of Ranvier in a nodal complex. The Neurofascin (Nfasc) gene encodes neuronal Neurofascin 186 (Nfasc186) at the node and glial Neurofascin 155 at the paranode, and these proteins play a key role in node assembly. However, their role in the maintenance and stability of the node is less well understood. Here we show that by inducible ablation of Nfasc in neurons in adult mice, Nfasc186 expression is reduced by >99% and 94% at PNS and CNS nodes, respectively. Gliomedin and NrCAM at PNS and brevican at CNS nodes are largely lost with neuronal neurofascin; however, Nav at nodes of Ranvier persist, albeit with ∼40% reduction in expression levels. βIV Spectrin, ankyrin G, and, to a lesser extent, the β1 subunit of the sodium channel, are less affected at the PNS node than in the CNS. Nevertheless, there is a 38% reduction in PNS conduction velocity. Loss of Nfasc186 provokes CNS paranodal disorganization, but this does not contribute to loss of Nav. These results show that Nav at PNS nodes are still maintained in a nodal complex when neuronal neurofascin is depleted, whereas the retention of nodal Nav in the CNS, despite more extensive dissolution of the complex, suggests a supportive role for the partially disrupted paranodal axoglial junction in selectively maintaining Nav at the CNS node.
Keywords: myelination; neurofascin; nodes of Ranvier; sodium channels.
Figures


References
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/S0896-6273(01)00294-X. - DOI - PubMed
-
- Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/S0960-9822(01)00680-7. - DOI - PubMed
-
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O'Malley HA, Bharucha V, Meadows LS, Knudsen GA, Vilaythong A, Noebels JL, Saunders TL, Scheuer T, Shrager P, Catterall WA, Isom LL. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
