Long-term maintenance of Na+ channels at nodes of Ranvier depends on glial contact mediated by gliomedin and NrCAM
- PMID: 24719088
- PMCID: PMC3983794
- DOI: 10.1523/JNEUROSCI.4752-13.2014
Long-term maintenance of Na+ channels at nodes of Ranvier depends on glial contact mediated by gliomedin and NrCAM
Abstract
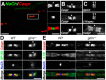
Clustering of Na(+) channels at the nodes of Ranvier is coordinated by myelinating glia. In the peripheral nervous system, axoglial contact at the nodes is mediated by the binding of gliomedin and glial NrCAM to axonal neurofascin 186 (NF186). This interaction is crucial for the initial clustering of Na(+) channels at heminodes. As a result, it is not clear whether continued axon-glial contact at nodes of Ranvier is required to maintain these channels at the nodal axolemma. Here, we report that, in contrast to mice that lack either gliomedin or NrCAM, absence of both molecules (and hence the glial clustering signal) resulted in a gradual loss of Na(+) channels and other axonal components from the nodes, the formation of binary nodes, and dysregulation of nodal gap length. Therefore, these mice exhibit neurological abnormalities and slower nerve conduction. Disintegration of the nodes occurred in an orderly manner, starting with the disappearance of neurofascin 186, followed by the loss of Na(+) channels and ankyrin G, and then βIV spectrin, a sequence that reflects the assembly of nodes during development. Finally, the absence of gliomedin and NrCAM led to the invasion of the outermost layer of the Schwann cell membrane beyond the nodal area and the formation of paranodal-like junctions at the nodal gap. Our results reveal that axon-glial contact mediated by gliomedin, NrCAM, and NF186 not only plays a role in Na(+) channel clustering during development, but also contributes to the long-term maintenance of Na(+) channels at nodes of Ranvier.
Keywords: NrCAM; Schwann; gliomedin; myelin; neurofascin; node of Ranvier.
Figures







References
-
- Bangratz M, Sarrazin N, Devaux J, Zambroni D, Echaniz-Laguna A, René F, Boërio D, Davoine CS, Fontaine B, Feltri ML, Benoit E, Nicole S. A mouse model of Schwartz-Jampel syndrome reveals myelinating Schwann cell dysfunction with persistent axonal depolarization in vitro and distal peripheral nerve hyperexcitability when perlecan is lacking. Am J Pathol. 2012;180:2040–2055. doi: 10.1016/j.ajpath.2012.01.035. - DOI - PMC - PubMed
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires Neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/S0896-6273(01)00294-X. - DOI - PubMed
-
- Brown AA, Xu T, Arroyo EJ, Levinson SR, Brophy PJ, Peles E, Scherer SS. Molecular organization of the nodal region is not altered in spontaneously diabetic BB-Wistar rats. J Neurosci Res. 2001;65:139–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
