Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice
- PMID: 24719095
- PMCID: PMC3983798
- DOI: 10.1523/JNEUROSCI.3445-12.2014
Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice
Abstract
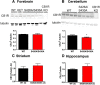
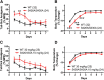
For many G-protein-coupled receptors (GPCRs), including cannabinoid receptor 1 (CB1R), desensitization has been proposed as a principal mechanism driving initial tolerance to agonists. GPCR desensitization typically requires phosphorylation by a G-protein-coupled receptor kinase (GRK) and interaction of the phosphorylated receptor with an arrestin. In simple model systems, CB1R is desensitized by GRK phosphorylation at two serine residues (S426 and S430). However, the role of these serine residues in tolerance and dependence for cannabinoids in vivo was unclear. Therefore, we generated mice where S426 and S430 were mutated to nonphosphorylatable alanines (S426A/S430A). S426A/S430A mutant mice were more sensitive to acutely administered delta-9-tetrahydrocannabinol (Δ(9)-THC), have delayed tolerance to Δ(9)-THC, and showed increased dependence for Δ(9)-THC. S426A/S430A mutants also showed increased responses to elevated levels of endogenous cannabinoids. CB1R desensitization in the periaqueductal gray and spinal cord following 7 d of treatment with Δ(9)-THC was absent in S426A/S430A mutants. Δ(9)-THC-induced downregulation of CB1R in the spinal cord was also absent in S426A/S430A mutants. Cultured autaptic hippocampal neurons from S426A/S430A mice showed enhanced endocannabinoid-mediated depolarization-induced suppression of excitation (DSE) and reduced agonist-mediated desensitization of DSE. These results indicate that S426 and S430 play major roles in the acute response to, tolerance to, and dependence on cannabinoids. Additionally, S426A/S430A mice are a novel model for studying pathophysiological processes thought to involve excessive endocannabinoid signaling such as drug addiction and metabolic disease. These mice also validate the approach of mutating GRK phosphorylation sites involved in desensitization as a general means to confer exaggerated signaling to GPCRs in vivo.
Keywords: CB1; GPCR; THC; cannabinoid; desensitization; tolerance.
Figures






References
-
- Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl) 2010;212:675–686. doi: 10.1007/s00213-010-1995-4. - DOI - PMC - PubMed
-
- Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
