Learning-induced plasticity regulates hippocampal sharp wave-ripple drive
- PMID: 24719097
- PMCID: PMC6609006
- DOI: 10.1523/JNEUROSCI.4288-13.2014
Learning-induced plasticity regulates hippocampal sharp wave-ripple drive
Abstract
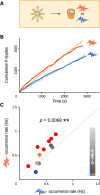
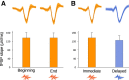
Hippocampal sharp wave-ripples (SPW-Rs) and associated place-cell reactivations are crucial for spatial memory consolidation during sleep and rest. However, it remains unclear how learning and consolidation requirements influence and regulate subsequent SPW-R activity. Indeed, SPW-R activity has been observed not only following complex behavioral tasks, but also after random foraging in familiar environments, despite markedly different learning requirements. Because transient increases in SPW-R rates have been reported following training on memory tasks, we hypothesized that SPW-R activity following learning (but not routine behavior) could involve specific regulatory processes related to ongoing consolidation. Interfering with ripples would then result in a dynamic compensatory response only when initial memory traces required consolidation. Here we trained rats on a spatial memory task, and showed that subsequent sleep periods where ripple activity was perturbed by timed electrical stimulation were indeed characterized by increased SPW-R occurrence rates compared with control sleep periods where stimulations were slightly delayed in time and did not interfere with ripples. Importantly, this did not occur following random foraging in a familiar environment. We next showed that this dynamic response was abolished following injection of an NMDA receptor blocker (MK-801) before, but not after training. Our results indicate that NMDA receptor-dependent processes occurring during learning, such as network "tagging" and plastic changes, regulate subsequent ripple-mediated consolidation of spatial memory during sleep.
Keywords: consolidation; hippocampus; learning; memory; regulation; ripples.
Figures
Similar articles
-
Most hippocampal CA1 pyramidal cells in rabbits increase firing during awake sharp-wave ripples and some do so in response to external stimulation and theta.J Neurophysiol. 2020 May 1;123(5):1671-1681. doi: 10.1152/jn.00056.2020. Epub 2020 Mar 25. J Neurophysiol. 2020. PMID: 32208887
-
Activity-dependent plasticity of mouse hippocampal assemblies in vitro.Front Neural Circuits. 2015 May 18;9:21. doi: 10.3389/fncir.2015.00021. eCollection 2015. Front Neural Circuits. 2015. PMID: 26041998 Free PMC article.
-
Disrupting neural activity related to awake-state sharp wave-ripple complexes prevents hippocampal learning.Front Behav Neurosci. 2012 Dec 4;6:84. doi: 10.3389/fnbeh.2012.00084. eCollection 2012. Front Behav Neurosci. 2012. PMID: 23316148 Free PMC article.
-
Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning.Hippocampus. 2015 Oct;25(10):1073-188. doi: 10.1002/hipo.22488. Hippocampus. 2015. PMID: 26135716 Free PMC article. Review.
-
Exploring Ripple Waves in the Human Brain.Clin EEG Neurosci. 2023 Nov;54(6):594-600. doi: 10.1177/15500594211034371. Epub 2021 Jul 21. Clin EEG Neurosci. 2023. PMID: 34287087 Review.
Cited by
-
Age Is Associated with Reduced Sharp-Wave Ripple Frequency and Altered Patterns of Neuronal Variability.J Neurosci. 2016 May 18;36(20):5650-60. doi: 10.1523/JNEUROSCI.3069-15.2016. J Neurosci. 2016. PMID: 27194342 Free PMC article.
-
Age-associated changes in waking hippocampal sharp-wave ripples.Hippocampus. 2020 Jan;30(1):28-38. doi: 10.1002/hipo.23005. Epub 2018 Nov 11. Hippocampus. 2020. PMID: 29981255 Free PMC article.
-
Rethinking retrosplenial cortex: Perspectives and predictions.Neuron. 2023 Jan 18;111(2):150-175. doi: 10.1016/j.neuron.2022.11.006. Epub 2022 Dec 1. Neuron. 2023. PMID: 36460006 Free PMC article. Review.
-
Spatial Learning Drives Rapid Goal Representation in Hippocampal Ripples without Place Field Accumulation or Goal-Oriented Theta Sequences.J Neurosci. 2022 May 11;42(19):3975-3988. doi: 10.1523/JNEUROSCI.2479-21.2022. Epub 2022 Apr 8. J Neurosci. 2022. PMID: 35396328 Free PMC article.
-
Brief targeted memory reactivation during the awake state enhances memory stability and benefits the weakest memories.Sci Rep. 2017 Nov 10;7(1):15325. doi: 10.1038/s41598-017-15608-x. Sci Rep. 2017. PMID: 29127388 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
