Ezh2 regulates adult hippocampal neurogenesis and memory
- PMID: 24719098
- PMCID: PMC6609005
- DOI: 10.1523/JNEUROSCI.4129-13.2014
Ezh2 regulates adult hippocampal neurogenesis and memory
Abstract
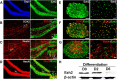
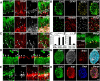
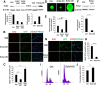
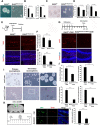
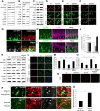
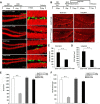
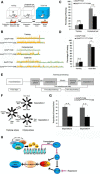
Adult neurogenesis is thought to be crucial for preserving cognitive functions, which is tightly controlled by various epigenetic regulators. As the methyltransferase of histone H3K27, the role of Ezh2 in neurogenesis of adult mice and its mechanism of action are largely unknown. Here, we show that Ezh2 is expressed in actively dividing neural stem cells (NSCs)/progenitor cells as well as mature neurons, but not in quiescent NSCs in the subgranular zone. The deletion of Ezh2 in NSCs/progenitor cells results in a reduction in progenitor cell proliferation. Furthermore, we found that Ezh2 regulates progenitor cell proliferation by suppressing Pten expression and promoting the activation of Akt-mTOR. Moreover, the loss of Ezh2 in progenitor cells leads to a decrease in the number of neurons, which was observed by long-term tracing. Strikingly, conditional knockout of Ezh2 ultimately results in impairments in spatial learning and memory, contextual fear memory, and pattern separation. Our findings demonstrate the essential role of Ezh2 in the proliferation of progenitor cells, thus providing insight into the molecular mechanisms of adult neurogenesis in preserving cognitive functions.
Keywords: Ezh2; adult neural stem cell; hippocampus; learning and memory; neurogenesis.
Figures

References
-
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1:3166–3173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
