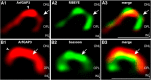
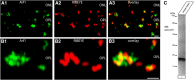
ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms an NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis
- PMID: 24719103
- PMCID: PMC6609000
- DOI: 10.1523/JNEUROSCI.3837-13.2014
ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms an NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis
Abstract
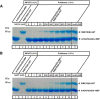
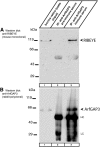
Ribbon synapses are tonically active synapses in the retina and inner ear with intense vesicle traffic. How this traffic is organized and regulated is still unknown. Synaptic ribbons, large presynaptic structures associated with numerous synaptic vesicles, appear to be essential for this process. The base of the synaptic ribbon is anchored at the active zone and is a hotspot of exocytosis. The synaptic ribbon complex is also important for vesicle replenishment. RIBEYE is a unique and major component of synaptic ribbons. It consists of a unique A-domain and an NAD(H)-binding, C-terminal B-domain. In the present study, we show that the Arf-GTPase activating protein-3 (ArfGAP3), a well characterized regulator of vesicle formation at the Golgi apparatus, is also a component of the synaptic ribbon complex in photoreceptor synapses of the mouse retina and interacts with RIBEYE as shown by multiple, independent approaches. ArfGAP3 binds to RIBEYE(B)-domain in an NAD(H)-dependent manner. The interaction is redox sensitive because NADH is more efficient than the oxidized NAD(+) in promoting ArfGAP3-RIBEYE interaction. RIBEYE competes with the GTP-binding protein Arf1 for binding to ArfGAP3. Thus, binding of RIBEYE(B) to ArfGAP3 could prevent inactivation of Arf1 by ArfGAP3 and provides the synaptic ribbon with the possibility to control Arf1 function. The interaction is relevant for endocytic vesicle trafficking because overexpression of ArfGAP3 in photoreceptors strongly inhibited endocytotic uptake of FM1-43.
Keywords: ArfGAP3; RIBEYE; endocytosis; photoreceptor synapse; ribbon synapse; synaptic ribbon.
Figures











Similar articles
-
Nicotinamide adenine dinucleotide-dependent binding of the neuronal Ca2+ sensor protein GCAP2 to photoreceptor synaptic ribbons.J Neurosci. 2010 May 12;30(19):6559-76. doi: 10.1523/JNEUROSCI.3701-09.2010. J Neurosci. 2010. PMID: 20463219 Free PMC article.
-
RIBEYE(B)-domain binds to lipid components of synaptic vesicles in an NAD(H)-dependent, redox-sensitive manner.Biochem J. 2017 Mar 20;474(7):1205-1220. doi: 10.1042/BCJ20160886. Biochem J. 2017. PMID: 28202712
-
Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons.J Neurosci. 2008 Aug 6;28(32):7954-67. doi: 10.1523/JNEUROSCI.1964-08.2008. J Neurosci. 2008. PMID: 18685021 Free PMC article.
-
Dual use of the transcriptional repressor (CtBP2)/ribbon synapse (RIBEYE) gene: how prevalent are multifunctional genes?Trends Neurosci. 2001 Oct;24(10):555-7. doi: 10.1016/s0166-2236(00)01894-4. Trends Neurosci. 2001. PMID: 11576649 Review.
-
The making of synaptic ribbons: how they are built and what they do.Neuroscientist. 2009 Dec;15(6):611-24. doi: 10.1177/1073858409340253. Neuroscientist. 2009. PMID: 19700740 Review.
Cited by
-
The Calcineurin-Binding, Activity-Dependent Splice Variant Dynamin1xb Is Highly Enriched in Synapses in Various Regions of the Central Nervous System.Front Mol Neurosci. 2017 Jul 25;10:230. doi: 10.3389/fnmol.2017.00230. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28790889 Free PMC article.
-
Rabconnectin-3α/DMXL2 Is Locally Enriched at the Synaptic Ribbon of Rod Photoreceptor Synapses.Cells. 2023 Jun 19;12(12):1665. doi: 10.3390/cells12121665. Cells. 2023. PMID: 37371135 Free PMC article.
-
Early Synapse-Specific Alterations of Photoreceptor Mitochondria in the EAE Mouse Model of Multiple Sclerosis.Cells. 2025 Jan 30;14(3):206. doi: 10.3390/cells14030206. Cells. 2025. PMID: 39936997 Free PMC article.
-
Ciliary Proteins Repurposed by the Synaptic Ribbon: Trafficking Myristoylated Proteins at Rod Photoreceptor Synapses.Int J Mol Sci. 2022 Jun 27;23(13):7135. doi: 10.3390/ijms23137135. Int J Mol Sci. 2022. PMID: 35806143 Free PMC article.
-
The cysteine proteome.Free Radic Biol Med. 2015 Jul;84:227-245. doi: 10.1016/j.freeradbiomed.2015.03.022. Epub 2015 Apr 3. Free Radic Biol Med. 2015. PMID: 25843657 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
