Auditory thalamus and auditory cortex are equally modulated by context during flexible categorization of sounds
- PMID: 24719107
- PMCID: PMC3983805
- DOI: 10.1523/JNEUROSCI.4888-13.2014
Auditory thalamus and auditory cortex are equally modulated by context during flexible categorization of sounds
Abstract
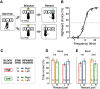
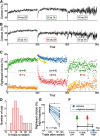
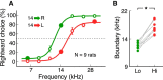
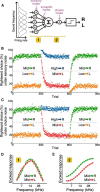
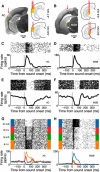
In a dynamic world, animals must adapt rapidly to changes in the meaning of environmental cues. Such changes can influence the neural representation of sensory stimuli. Previous studies have shown that associating a stimulus with a reward or punishment can modulate neural activity in the auditory cortex (AC) and its thalamic input, the medial geniculate body (MGB). However, it is not known whether changes in stimulus-action associations alone can also modulate neural responses in these areas. We designed a categorization task for rats in which the boundary that separated low- from high-frequency sounds varied several times within a behavioral session, thus allowing us to manipulate the action associated with some sounds without changing the associated reward. We developed a computational model that accounted for the rats' performance and compared predictions from this model with sound-evoked responses from single neurons in AC and MGB in animals performing this task. We found that the responses of 15% of AC neurons and 16% of MGB neurons were modulated by changes in stimulus-action association and that the magnitude of the modulation was comparable between the two brain areas. Our results suggest that the AC and thalamus play only a limited role in mediating changes in associations between acoustic stimuli and behavioral responses.
Keywords: auditory; categorization; flexibility; reward; stimulus–action association.
Figures



Similar articles
-
A comparison of neural responses in the primary auditory cortex, amygdala, and medial prefrontal cortex of cats during auditory discrimination tasks.J Neurophysiol. 2019 Mar 1;121(3):785-798. doi: 10.1152/jn.00425.2018. Epub 2019 Jan 16. J Neurophysiol. 2019. PMID: 30649979
-
Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat.PLoS One. 2010 Nov 19;5(11):e14071. doi: 10.1371/journal.pone.0014071. PLoS One. 2010. PMID: 21124913 Free PMC article.
-
Auditory cortex reflects goal-directed movement but is not necessary for behavioral adaptation in sound-cued reward tracking.J Neurophysiol. 2020 Oct 1;124(4):1056-1071. doi: 10.1152/jn.00736.2019. Epub 2020 Aug 26. J Neurophysiol. 2020. PMID: 32845769
-
Corticofugal modulation of the auditory thalamus.Exp Brain Res. 2003 Dec;153(4):579-90. doi: 10.1007/s00221-003-1680-5. Epub 2003 Oct 22. Exp Brain Res. 2003. PMID: 14574430 Review.
-
An overview of stimulus-specific adaptation in the auditory thalamus.Brain Topogr. 2014 Jul;27(4):480-99. doi: 10.1007/s10548-013-0342-6. Epub 2013 Dec 17. Brain Topogr. 2014. PMID: 24343247 Review.
Cited by
-
The Contribution of Primary Auditory Cortex to Auditory Categorization in Behaving Monkeys.Front Neurosci. 2018 Aug 29;12:601. doi: 10.3389/fnins.2018.00601. eCollection 2018. Front Neurosci. 2018. PMID: 30210282 Free PMC article.
-
Representation of Auditory Task Components and of Their Relationships in Primate Auditory Cortex.Front Neurosci. 2020 Apr 21;14:306. doi: 10.3389/fnins.2020.00306. eCollection 2020. Front Neurosci. 2020. PMID: 32372903 Free PMC article.
-
Locomotion and Task Demands Differentially Modulate Thalamic Audiovisual Processing during Active Search.Curr Biol. 2015 Jul 20;25(14):1885-91. doi: 10.1016/j.cub.2015.05.045. Epub 2015 Jun 25. Curr Biol. 2015. PMID: 26119749 Free PMC article.
-
Parallel processing by cortical inhibition enables context-dependent behavior.Nat Neurosci. 2017 Jan;20(1):62-71. doi: 10.1038/nn.4436. Epub 2016 Oct 31. Nat Neurosci. 2017. PMID: 27798631 Free PMC article.
-
Associations between sounds and actions in early auditory cortex of nonhuman primates.Elife. 2019 Apr 4;8:e43281. doi: 10.7554/eLife.43281. Elife. 2019. PMID: 30946010 Free PMC article.
References
-
- Albanese D, Visintainer R, Merler S, Riccadonna S, Jurman G, Furlanello C. mlpy: Machine Learning Python. 2012. Retrieved Feb. 5, 2014, from http://arxiv.org/abs/1202.6548.
-
- Bishop CM. Neural networks for pattern recognition. Oxford: OUP; 1995.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
