The Drosophila auditory system
- PMID: 24719289
- PMCID: PMC4007284
- DOI: 10.1002/wdev.128
The Drosophila auditory system
Abstract
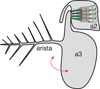
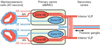
Development of a functional auditory system in Drosophila requires specification and differentiation of the chordotonal sensilla of Johnston's organ (JO) in the antenna, correct axonal targeting to the antennal mechanosensory and motor center in the brain, and synaptic connections to neurons in the downstream circuit. Chordotonal development in JO is functionally complicated by structural, molecular, and functional diversity that is not yet fully understood, and construction of the auditory neural circuitry is only beginning to unfold. Here, we describe our current understanding of developmental and molecular mechanisms that generate the exquisite functions of the Drosophila auditory system, emphasizing recent progress and highlighting important new questions arising from research on this remarkable sensory system.
© 2013 Wiley Periodicals, Inc.
Conflict of interest statement
Grace Boekhoff-Falk, No conflict of interest.
Daniel F. Eberl, No conflict of interest.
Figures






References
-
- Hoy RR. Acute as a bug's ear: an informal discussion of hearing in insects. In: Hoy RR, Popper AN, Fay RR, editors. Comparative Hearing: Insects. Vol. 10. New York: Springer; 1998. pp. 1–17.
-
- Eberl DF. Feeling the vibes: chordotonal mechanisms in insect hearing. Curr. Opin. Neurobiol. 1999;9:389–393. - PubMed
-
- Field LH, Matheson T. Chordotonal organs of insects. In: Evans PD, editor. Adv. Insect Physiol. Vol. 27. San Diego: Academic Press; 1998. pp. 1–228.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

