Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain
- PMID: 24719311
- PMCID: PMC4314689
- DOI: 10.1002/art.38656
Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain
Abstract
Objective: To investigate the role of the sensory neuropeptide calcitonin gene-related peptide (CGRP) in peripheral sensitization in experimental models of osteoarthritis (OA) pain.
Methods: Experimental knee OA was induced in rats by intraarticular injection of monosodium iodoacetate (MIA) or by transection of the medial meniscus (MMT). Single-unit recordings of joint-innervating nociceptors were obtained in MIA- and saline-treated rats following administration of CGRP or the CGRP receptor antagonist CGRP 8-37. Effects of CGRP 8-37 were also examined in rats that underwent MMT and sham operations. Protein and messenger RNA (mRNA) levels of CGRP receptor components in the L3-L4 dorsal root ganglion (DRG) were investigated following MIA treatment.
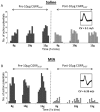
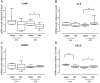
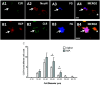
Results: In both the MIA and MMT groups, the mechanical sensitivity of joint nociceptors was enhanced compared to that in the control groups. Exogenous CGRP increased mechanical sensitivity in a greater proportion of joint nociceptors in the MIA-treated rats than in the saline-treated rats. Local blockade of endogenous CGRP by CGRP 8-37 reversed both the MIA- and MMT-induced enhancement of joint nociceptor responses. Joint afferent cell bodies coexpressed the receptor for CGRP, called the calcitonin-like receptor (CLR), and the intracellular accessory CGRP receptor component protein. MIA treatment increased the levels of mRNA for CLR in the L3-L4 DRG and the levels of CLR protein in medium and large joint afferent neurons.
Conclusion: Our findings provide new and compelling evidence implicating a role of CGRP in peripheral sensitization in experimental OA. Our novel finding of CGRP-mediated control of joint nociceptor mechanosensitivity suggests that the CGRP receptor system may be an important target for the modulation of pain during OA. CGRP receptor antagonists recently developed for migraine pain should be investigated for their efficacy against pain in OA.
© 2014 The Authors. Arthritis & Rheumatology is published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures


References
-
- Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–73. - PubMed
-
- Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. - PubMed
-
- Creamer P, Hunt M, Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intraarticular anesthetic. J Rheumatol. 1996;23:1031–6. - PubMed
-
- Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26:773–7. - PubMed
-
- Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

