Dennd3 functions as a guanine nucleotide exchange factor for small GTPase Rab12 in mouse embryonic fibroblasts
- PMID: 24719330
- PMCID: PMC4022869
- DOI: 10.1074/jbc.M113.546689
Dennd3 functions as a guanine nucleotide exchange factor for small GTPase Rab12 in mouse embryonic fibroblasts
Abstract
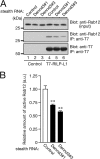
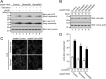
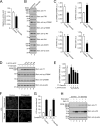
Small GTPase Rab12 regulates mTORC1 (mammalian target of rapamycin complex 1) activity and autophagy through controlling PAT4 (proton/amino acid transporter 4) trafficking from recycling endosomes to lysosomes, where PAT4 is degraded. However, the precise regulatory mechanism of the Rab12-mediated membrane trafficking pathway remained to be determined because a physiological Rab12-GEF (guanine nucleotide exchange factor) had yet to be identified. In this study we performed functional analyses of Dennd3, which has recently been shown to possess a GEF activity toward Rab12 in vitro. The results showed that knockdown of Dennd3 in mouse embryonic fibroblast cells caused an increase in the amount of PAT4 protein, the same as Rab12 knockdown did, and knockdown of Dennd3 and overexpression of Dennd3 were found to result in an increase and a decrease, respectively, in the intracellular amino acid concentration. Dennd3 overexpression was also found to reduce mTORC1 activity and promoted autophagy in a Rab12-dependent manner. Unexpectedly, however, Dennd3 knockdown had no effect on mTORC1 activity or autophagy despite increasing the intracellular amino acid concentration. Further study showed that Dennd3 knockdown reduced Akt activity, and the reduction in Akt activity is likely to have canceled out amino acid-induced mTORC1 activation through PAT4. These findings indicated that Dennd3 not only functions as a Rab12-GEF but also modulates Akt signaling in mouse embryonic fibroblast cells.
Keywords: Amino Acids Transport; Autophagy; Endosomes; G Proteins; Guanine Nucleotide Exchange Factor (GEF); Low Molecular Weight G Proteins; Membrane Trafficking; Rab Proteins; mTOR Complex (mTORC).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

