Enhanced immunogenicity of an HIV-1 DNA vaccine delivered with electroporation via combined intramuscular and intradermal routes
- PMID: 24719412
- PMCID: PMC4054344
- DOI: 10.1128/JVI.00183-14
Enhanced immunogenicity of an HIV-1 DNA vaccine delivered with electroporation via combined intramuscular and intradermal routes
Abstract
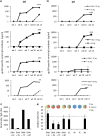
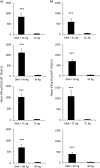
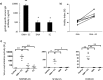
It is accepted that an effective prophylactic HIV-1 vaccine is likely to have the greatest impact on viral transmission rates. As previous reports have implicated DNA-priming, protein boost regimens to be efficient activators of humoral responses, we sought to optimize this regimen to further augment vaccine immunogenicity. Here we evaluated single versus concurrent intradermal (i.d.) and intramuscular (i.m.) vaccinations as a DNA-priming strategy for their abilities to elicit humoral and cellular responses against a model HIV-1 vaccine antigen, CN54-gp140. To further augment vaccine-elicited T and B cell responses, we enhanced cellular transfection with electroporation and then boosted the DNA-primed responses with homologous protein delivered subcutaneously (s.c.), intranasally (i.n.), i.m., or transcutaneously (t.c.). In mice, the concurrent priming regimen resulted in significantly elevated gamma interferon T cell responses and high-avidity antigen-specific IgG B cell responses, a hallmark of B cell maturation. Protein boosting of the concurrent DNA strategy further enhanced IgG concentrations but had little impact on T cell reactivity. Interestingly protein boosting by the subcutaneous route increased antibody avidity to a greater extent than protein boosting by either the i.m., i.n., or t.c. route, suggesting that this route may be preferential for driving B cell maturation. Using an alternative and larger animal model, the rabbit, we found the concurrent DNA-priming strategy followed by s.c. protein boosting to again be capable of eliciting high-avidity humoral responses and to also be able to neutralize HIV-1 pseudoviruses from diverse clades (clades A, B, and C). Taken together, we show that concurrent multiple-route DNA vaccinations induce strong cellular immunity, in addition to potent and high-avidity humoral immune responses.
Importance: The route of vaccination has profound effects on prevailing immune responses. Due to the insufficient immunogenicity and protection of current DNA delivery strategies, we evaluated concurrent DNA delivery via simultaneous administration of plasmid DNA by the i.m. and i.d. routes. The rationale behind this study was to provide clear evidence of the utility of concurrent vaccinations for an upcoming human clinical trial. Furthermore, this work will guide future preclinical studies by evaluating the use of model antigens and plasmids for prime-boost strategies. This paper will be of interest not only to virologists and vaccinologists working in the HIV field but also to researchers working in other viral vaccine settings and, critically, to the wider field of vaccine delivery.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Combined Skin and Muscle DNA Priming Provides Enhanced Humoral Responses to a Human Immunodeficency Virus Type 1 Clade C Envelope Vaccine.Hum Gene Ther. 2018 Sep;29(9):1011-1028. doi: 10.1089/hum.2018.075. Hum Gene Ther. 2018. PMID: 30027768 Free PMC article. Clinical Trial.
-
HIV-DNA Given with or without Intradermal Electroporation Is Safe and Highly Immunogenic in Healthy Swedish HIV-1 DNA/MVA Vaccinees: A Phase I Randomized Trial.PLoS One. 2015 Jun 29;10(6):e0131748. doi: 10.1371/journal.pone.0131748. eCollection 2015. PLoS One. 2015. PMID: 26121679 Free PMC article. Clinical Trial.
-
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost.J Virol. 2019 Jan 17;93(3):e01529-18. doi: 10.1128/JVI.01529-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429343 Free PMC article.
-
Therapeutic and prophylactic DNA vaccines for HIV-1.Expert Opin Biol Ther. 2013 Apr;13(4):563-73. doi: 10.1517/14712598.2013.758709. Expert Opin Biol Ther. 2013. PMID: 23477730 Review.
-
Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models.Expert Rev Vaccines. 2017 Oct;16(10):973-985. doi: 10.1080/14760584.2017.1371594. Epub 2017 Sep 4. Expert Rev Vaccines. 2017. PMID: 28838267 Free PMC article. Review.
Cited by
-
What We Learned about the Feasibility of Gene Electrotransfer for Vaccination on a Model of COVID-19 Vaccine.Pharmaceutics. 2023 Jul 19;15(7):1981. doi: 10.3390/pharmaceutics15071981. Pharmaceutics. 2023. PMID: 37514166 Free PMC article.
-
Combined Skin and Muscle DNA Priming Provides Enhanced Humoral Responses to a Human Immunodeficency Virus Type 1 Clade C Envelope Vaccine.Hum Gene Ther. 2018 Sep;29(9):1011-1028. doi: 10.1089/hum.2018.075. Hum Gene Ther. 2018. PMID: 30027768 Free PMC article. Clinical Trial.
-
Poly(Lactic Acid) Nanoparticles Targeting α5β1 Integrin as Vaccine Delivery Vehicle, a Prospective Study.PLoS One. 2016 Dec 14;11(12):e0167663. doi: 10.1371/journal.pone.0167663. eCollection 2016. PLoS One. 2016. PMID: 27973577 Free PMC article.
-
Modulation of Vaccine-Induced CD4 T Cell Functional Profiles by Changes in Components of HIV Vaccine Regimens in Humans.J Virol. 2018 Nov 12;92(23):e01143-18. doi: 10.1128/JVI.01143-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209165 Free PMC article.
-
Blocking T-cell egress with FTY720 extends DNA vaccine expression but reduces immunogenicity.Immunology. 2022 Mar;165(3):301-311. doi: 10.1111/imm.13429. Epub 2021 Dec 12. Immunology. 2022. PMID: 34775601 Free PMC article.
References
-
- Martinon F, Kaldma K, Sikut R, Culina S, Romain G, Tuomela M, Adojaan M, Mannik A, Toots U, Kivisild T, Morin J, Brochard P, Delache B, Tripiciano A, Ensoli F, Stanescu I, Le Grand R, Ustav M. 2009. Persistent immune responses induced by a human immunodeficiency virus DNA vaccine delivered in association with electroporation in the skin of nonhuman primates. Hum. Gene Ther. 20:1291–1307. 10.1089/hum.2009.044 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

