NeuroD1 mediates nicotine-induced migration and invasion via regulation of the nicotinic acetylcholine receptor subunits in a subset of neural and neuroendocrine carcinomas
- PMID: 24719457
- PMCID: PMC4038504
- DOI: 10.1091/mbc.E13-06-0316
NeuroD1 mediates nicotine-induced migration and invasion via regulation of the nicotinic acetylcholine receptor subunits in a subset of neural and neuroendocrine carcinomas
Abstract
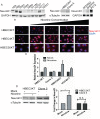
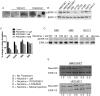
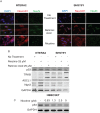
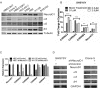
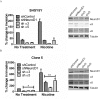
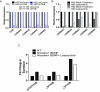
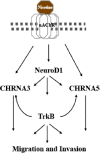
Cigarette smoking is a major risk factor for acquisition of small cell lung cancer (SCLC). A role has been demonstrated for the basic helix-loop-helix transcription factor NeuroD1 in the pathogenesis of neural and neuroendocrine lung cancer, including SCLC. In the present study we investigate the possible function of NeuroD1 in established tumors, as well as actions early on in pathogenesis, in response to nicotine. We demonstrate that nicotine up-regulates NeuroD1 in immortalized normal bronchial epithelial cells and a subset of undifferentiated carcinomas. Increased expression of NeuroD1 subsequently leads to regulation of expression and function of the nicotinic acetylcholine receptor subunit cluster of α3, α5, and β4. In addition, we find that coordinated expression of these subunits by NeuroD1 leads to enhanced nicotine-induced migration and invasion, likely through changes in intracellular calcium. These findings suggest that aspects of the pathogenesis of neural and neuroendocrine lung cancers may be affected by a nicotine- and NeuroD1-induced positive feedback loop.
© 2014 Osborne et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

Similar articles
-
NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM.Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6524-9. doi: 10.1073/pnas.1303932110. Epub 2013 Apr 3. Proc Natl Acad Sci U S A. 2013. PMID: 23553831 Free PMC article.
-
ASCL1 regulates the expression of the CHRNA5/A3/B4 lung cancer susceptibility locus.Mol Cancer Res. 2010 Feb;8(2):194-203. doi: 10.1158/1541-7786.MCR-09-0185. Epub 2010 Feb 2. Mol Cancer Res. 2010. PMID: 20124469 Free PMC article.
-
Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells.Pulm Pharmacol Ther. 2007;20(6):629-41. doi: 10.1016/j.pupt.2006.07.001. Epub 2006 Aug 18. Pulm Pharmacol Ther. 2007. PMID: 17015027
-
Nicotine-induced resistance of non-small cell lung cancer to treatment--possible mechanisms.Postepy Hig Med Dosw (Online). 2016 Mar 4;70:186-93. doi: 10.5604/17322693.1196391. Postepy Hig Med Dosw (Online). 2016. PMID: 26943316 Review.
-
Nicotine, lung and cancer.Anticancer Agents Med Chem. 2007 Jul;7(4):461-6. doi: 10.2174/187152007781058587. Anticancer Agents Med Chem. 2007. PMID: 17630920 Review.
Cited by
-
ASCL1, NKX2-1, and PROX1 co-regulate subtype-specific genes in small-cell lung cancer.iScience. 2021 Aug 5;24(9):102953. doi: 10.1016/j.isci.2021.102953. eCollection 2021 Sep 24. iScience. 2021. PMID: 34466783 Free PMC article.
-
Nucleosome Repositioning: A Novel Mechanism for Nicotine- and Cocaine-Induced Epigenetic Changes.PLoS One. 2015 Sep 28;10(9):e0139103. doi: 10.1371/journal.pone.0139103. eCollection 2015. PLoS One. 2015. PMID: 26414157 Free PMC article.
-
Transcriptional deregulation underlying the pathogenesis of small cell lung cancer.Transl Lung Cancer Res. 2018 Feb;7(1):4-20. doi: 10.21037/tlcr.2017.10.07. Transl Lung Cancer Res. 2018. PMID: 29535909 Free PMC article. Review.
-
New Approaches to SCLC Therapy: From the Laboratory to the Clinic.J Thorac Oncol. 2020 Apr;15(4):520-540. doi: 10.1016/j.jtho.2020.01.016. Epub 2020 Feb 1. J Thorac Oncol. 2020. PMID: 32018053 Free PMC article. Review.
-
NeuroD1 promotes tumor cell proliferation and tumorigenesis by directly activating the pentose phosphate pathway in colorectal carcinoma.Oncogene. 2021 Dec;40(50):6736-6747. doi: 10.1038/s41388-021-02063-2. Epub 2021 Oct 16. Oncogene. 2021. PMID: 34657129
References
-
- Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–2020. - PubMed
-
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

