Differences in mouse and human nonmemory B cell pools
- PMID: 24719464
- PMCID: PMC4046845
- DOI: 10.4049/jimmunol.1300692
Differences in mouse and human nonmemory B cell pools
Abstract
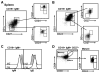
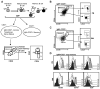
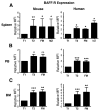
Identifying cross-species similarities and differences in immune development and function is critical for maximizing the translational potential of animal models. Coexpression of CD21 and CD24 distinguishes transitional and mature B cell subsets in mice. In this study, we validate these markers for identifying analogous subsets in humans and use them to compare the nonmemory B cell pools in mice and humans, across tissues, and during fetal/neonatal and adult life. Among human CD19(+)IgM(+) B cells, the CD21/CD24 schema identifies distinct populations that correspond to transitional 1 (T1), transitional 2 (T2), follicular mature, and marginal zone subsets identified in mice. Markers specific to human B cell development validate the identity of marginal zone cells and the maturation status of human CD21/CD24 nonmemory B cell subsets. A comparison of the nonmemory B cell pools in bone marrow, blood, and spleen in mice and humans shows that transitional B cells comprise a much smaller fraction in adult humans than mice. T1 cells are a major contributor to the nonmemory B cell pool in mouse bone marrow, in which their frequency is more than twice that in humans. Conversely, in spleen, the T1:T2 ratio shows that T2 cells are proportionally ∼ 8-fold higher in humans than in mice. Despite the relatively small contribution of transitional B cells to the human nonmemory pool, the number of naive follicular mature cells produced per transitional B cell is 3- to 6-fold higher across tissues than in mice. These data suggest differing dynamics or mechanisms produce the nonmemory B cell compartments in mice and humans.
Figures



References
-
- Vossenkamper A, Spencer J. Transitional B cells: how well are the checkpoints for specificity understood? Arch Immunol Ther Exp (Warsz) 2011;59:379–384. - PubMed
-
- Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. - PubMed
-
- Srivastava B, Lindsley RC, Nikbakht N, Allman D. Models for peripheral B cell development and homeostasis. Semin Immunol. 2005;17:175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

