Comparison of Heavy Labeled (SIL) Peptide versus SILAC Protein Internal Standards for LC-MS/MS Quantification of Hepatic Drug Transporters
- PMID: 24719762
- PMCID: PMC3955635
- DOI: 10.1155/2014/451510
Comparison of Heavy Labeled (SIL) Peptide versus SILAC Protein Internal Standards for LC-MS/MS Quantification of Hepatic Drug Transporters
Abstract
We studied the precision of quantification of organic anion-transporting polypeptide 1B1 (OATP1B1), OATP1B3, OATP2B1, and P-glycoprotein (P-gp) in human livers by surrogate peptide based LC-MS/MS approach using two different internal standards: stable isotope labeled peptide (SIL) versus stable isotope labeled protein (SILAC). The SIL peptides were procured commercially and the SILAC proteins were generated in-house by labeling arginine and/or lysine residues in cells expressing these transporters. Liver tissue (n = 20) was homogenized and the membrane fraction was isolated. The membranes were trypsin digested and the peptides were analyzed using LC-MS/MS under optimized conditions. The precision in the quantification of proteins in three independently trypsin digested samples from each liver was calculated as the standard deviation of the log transformed protein concentration. The precision of the SIL internal standard method was either slightly (P < 0.05, paired t-test) better than that of the SILAC method (OATP1B1, OATP1B3, and P-gp) or not different (OATP2B1). Trypsin digestion, as measured by the response of the labeled peptide derived from the SILAC protein, was consistent across liver samples. These results indicate that when maximum trypsin digestion is ensured, the SIL internal standard method can be used with confidence for quantification of drug transporters.
Figures

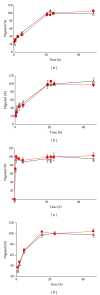
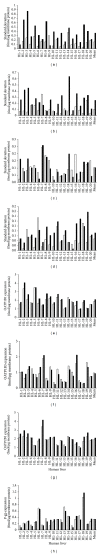
Similar articles
-
Comparison of a stable isotope labeled (SIL) peptide and an extended SIL peptide as internal standards to track digestion variability of an unstable signature peptide during quantification of a cancer biomarker, human osteopontin, from plasma using capillary microflow LC-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Sep 15;1001:156-68. doi: 10.1016/j.jchromb.2015.05.040. Epub 2015 Jul 26. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 26279007
-
Development of a multiplex UPLC-MRM MS method for quantification of human membrane transport proteins OATP1B1, OATP1B3 and OATP2B1 in in vitro systems and tissues.Anal Chim Acta. 2012 Mar 2;717:67-76. doi: 10.1016/j.aca.2011.12.005. Epub 2011 Dec 24. Anal Chim Acta. 2012. PMID: 22304817
-
High-sensitivity LC-MS/MS quantification of peptides and proteins in complex biological samples: the impact of enzymatic digestion and internal standard selection on method performance.Anal Chem. 2013 Oct 15;85(20):9528-35. doi: 10.1021/ac4015116. Epub 2013 Sep 25. Anal Chem. 2013. PMID: 24010948
-
LC-MS/MS determination of a human mAb drug candidate in rat serum using an isotopically labeled universal mAb internal standard.J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Feb 15;1044-1045:166-176. doi: 10.1016/j.jchromb.2016.12.044. Epub 2017 Jan 4. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 28113139
-
Internal standards in the quantitative determination of protein biopharmaceuticals using liquid chromatography coupled to mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Apr 15;893-894:1-14. doi: 10.1016/j.jchromb.2012.02.021. Epub 2012 Feb 21. J Chromatogr B Analyt Technol Biomed Life Sci. 2012. PMID: 22426285 Review.
Cited by
-
Abundance of Drug Transporters in the Human Kidney Cortex as Quantified by Quantitative Targeted Proteomics.Drug Metab Dispos. 2016 Dec;44(12):1920-1924. doi: 10.1124/dmd.116.072066. Epub 2016 Sep 12. Drug Metab Dispos. 2016. PMID: 27621205 Free PMC article.
-
Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3-O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans.Drug Metab Dispos. 2018 Apr;46(4):367-379. doi: 10.1124/dmd.117.078428. Epub 2018 Jan 17. Drug Metab Dispos. 2018. PMID: 29343609 Free PMC article.
-
The Promises of Quantitative Proteomics in Precision Medicine.J Pharm Sci. 2017 Mar;106(3):738-744. doi: 10.1016/j.xphs.2016.11.017. Epub 2016 Dec 8. J Pharm Sci. 2017. PMID: 27939376 Free PMC article.
-
Developmental Regulation of Drug-Processing Genes in Livers of Germ-Free Mice.Toxicol Sci. 2015 Sep;147(1):84-103. doi: 10.1093/toxsci/kfv110. Epub 2015 Jun 1. Toxicol Sci. 2015. PMID: 26032512 Free PMC article.
-
Optimized approaches for quantification of drug transporters in tissues and cells by MRM proteomics.AAPS J. 2014 Jul;16(4):634-48. doi: 10.1208/s12248-014-9602-y. Epub 2014 Apr 22. AAPS J. 2014. PMID: 24752720 Free PMC article. Review.
References
-
- Kamiie J, Ohtsuki S, Iwase R, et al. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharmaceutical Research. 2008;25(6):1469–1483. - PubMed
-
- Sakamoto A, Matsumaru T, Ishiguro N, et al. Reliability and robustness of simultaneous absolute quantification of drug transporters, cytochrome P450 enzymes, and Udp-glucuronosyltransferases in human liver tissue by multiplexed MRM/selected reaction monitoring mode tandem mass spectrometry with nano-liquid chromatography. Journal of Pharmaceutical Sciences. 2011;100(9):4037–4043. - PubMed
-
- Balogh LM, Kimoto E, Chupka J, Zhang H, Lai : Y. Membrane protein quantification by peptide-based mass spectrometry approaches: studies on the organic anion-transporting polypeptide family. Journal of Proteomics & Bioinformatics. 2012;84:1–8.
-
- Li N, Nemirovskiy OV, Zhang Y, et al. Absolute quantification of multidrug resistance-associated protein 2 (MRP2/ABCC2) using liquid chromatography tandem mass spectrometry. Analytical Biochemistry. 2008;380(2):211–222. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
