Comparison of Heavy Labeled (SIL) Peptide versus SILAC Protein Internal Standards for LC-MS/MS Quantification of Hepatic Drug Transporters
- PMID: 24719762
- PMCID: PMC3955635
- DOI: 10.1155/2014/451510
Comparison of Heavy Labeled (SIL) Peptide versus SILAC Protein Internal Standards for LC-MS/MS Quantification of Hepatic Drug Transporters
Abstract
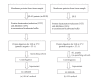
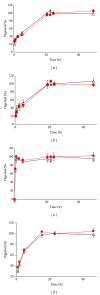
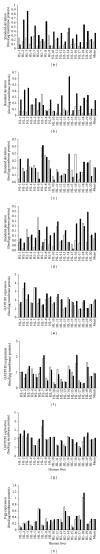
We studied the precision of quantification of organic anion-transporting polypeptide 1B1 (OATP1B1), OATP1B3, OATP2B1, and P-glycoprotein (P-gp) in human livers by surrogate peptide based LC-MS/MS approach using two different internal standards: stable isotope labeled peptide (SIL) versus stable isotope labeled protein (SILAC). The SIL peptides were procured commercially and the SILAC proteins were generated in-house by labeling arginine and/or lysine residues in cells expressing these transporters. Liver tissue (n = 20) was homogenized and the membrane fraction was isolated. The membranes were trypsin digested and the peptides were analyzed using LC-MS/MS under optimized conditions. The precision in the quantification of proteins in three independently trypsin digested samples from each liver was calculated as the standard deviation of the log transformed protein concentration. The precision of the SIL internal standard method was either slightly (P < 0.05, paired t-test) better than that of the SILAC method (OATP1B1, OATP1B3, and P-gp) or not different (OATP2B1). Trypsin digestion, as measured by the response of the labeled peptide derived from the SILAC protein, was consistent across liver samples. These results indicate that when maximum trypsin digestion is ensured, the SIL internal standard method can be used with confidence for quantification of drug transporters.
Figures

References
-
- Kamiie J, Ohtsuki S, Iwase R, et al. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharmaceutical Research. 2008;25(6):1469–1483. - PubMed
-
- Sakamoto A, Matsumaru T, Ishiguro N, et al. Reliability and robustness of simultaneous absolute quantification of drug transporters, cytochrome P450 enzymes, and Udp-glucuronosyltransferases in human liver tissue by multiplexed MRM/selected reaction monitoring mode tandem mass spectrometry with nano-liquid chromatography. Journal of Pharmaceutical Sciences. 2011;100(9):4037–4043. - PubMed
-
- Balogh LM, Kimoto E, Chupka J, Zhang H, Lai : Y. Membrane protein quantification by peptide-based mass spectrometry approaches: studies on the organic anion-transporting polypeptide family. Journal of Proteomics & Bioinformatics. 2012;84:1–8.
-
- Li N, Nemirovskiy OV, Zhang Y, et al. Absolute quantification of multidrug resistance-associated protein 2 (MRP2/ABCC2) using liquid chromatography tandem mass spectrometry. Analytical Biochemistry. 2008;380(2):211–222. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
