Chronic fluoxetine treatment suppresses plasticity (long-term potentiation) in the mature rodent primary auditory cortex in vivo
- PMID: 24719772
- PMCID: PMC3956292
- DOI: 10.1155/2014/571285
Chronic fluoxetine treatment suppresses plasticity (long-term potentiation) in the mature rodent primary auditory cortex in vivo
Abstract
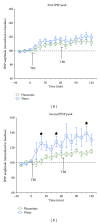
Several recent studies have provided evidence that chronic treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine can facilitate synaptic plasticity (e.g., ocular dominance shifts) in the adult central nervous system. Here, we assessed whether fluoxetine enhances long-term potentiation (LTP) in the thalamocortical auditory system of mature rats, a developmentally regulated form of plasticity that shows a characteristic decline during postnatal life. Adult rats were chronically treated with fluoxetine (administered in the drinking water, 0.2 mg/mL, four weeks of treatment). Electrophysiological assessments were conducted using an anesthetized (urethane) in vivo preparation, with LTP of field potentials in the primary auditory cortex (A1) induced by theta-burst stimulation of the medial geniculate nucleus. We find that, compared to water-treated control animals, fluoxetine-treated rats did not express higher levels of LTP and, in fact, exhibited reduced levels of potentiation at presumed intracortical A1 synapses. Bioactivity of fluoxetine was confirmed by a reduction of weight gain and fluid intake during the four-week treatment period. We conclude that chronic fluoxetine treatment fails to enhance LTP in the mature rodent thalamocortical auditory system, results that bring into question the notion that SSRIs act as general facilitators of synaptic plasticity in the mammalian forebrain.
Figures



Similar articles
-
Gating of long-term potentiation (LTP) in the thalamocortical auditory system of rats by serotonergic (5-HT) receptors.Brain Res. 2018 Mar 15;1683:1-11. doi: 10.1016/j.brainres.2018.01.004. Epub 2018 Jan 8. Brain Res. 2018. PMID: 29325855
-
Continuous white noise exposure during and after auditory critical period differentially alters bidirectional thalamocortical plasticity in rat auditory cortex in vivo.Eur J Neurosci. 2007 Nov;26(9):2576-84. doi: 10.1111/j.1460-9568.2007.05857.x. Epub 2007 Oct 26. Eur J Neurosci. 2007. PMID: 17970743
-
Decline of long-term potentiation (LTP) in the rat auditory cortex in vivo during postnatal life: involvement of NR2B subunits.Brain Res. 2009 Aug 4;1283:25-33. doi: 10.1016/j.brainres.2009.06.001. Epub 2009 Jun 9. Brain Res. 2009. PMID: 19520065
-
Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any?Curr Med Chem. 1999 Jun;6(6):469-80. Curr Med Chem. 1999. PMID: 10213794 Review.
-
Fluoxetine during pregnancy: impact on fetal development.Reprod Fertil Dev. 2005;17(6):641-50. doi: 10.1071/rd05030. Reprod Fertil Dev. 2005. PMID: 16263070 Review.
Cited by
-
Single fluoxetine treatment before but not after stress prevents stress-induced hippocampal long-term depression and spatial memory retrieval impairment in rats.Sci Rep. 2015 Jul 28;5:12667. doi: 10.1038/srep12667. Sci Rep. 2015. PMID: 26218751 Free PMC article.
-
Chronic Fluoxetine Induces the Enlargement of Perforant Path-Granule Cell Synapses in the Mouse Dentate Gyrus.PLoS One. 2016 Jan 20;11(1):e0147307. doi: 10.1371/journal.pone.0147307. eCollection 2016. PLoS One. 2016. PMID: 26788851 Free PMC article.
-
Effects of Fluoxetine and Visual Experience on Glutamatergic and GABAergic Synaptic Proteins in Adult Rat Visual Cortex.eNeuro. 2016 Jan 4;2(6):ENEURO.0126-15.2015. doi: 10.1523/ENEURO.0126-15.2015. eCollection 2015 Nov-Dec. eNeuro. 2016. PMID: 26730408 Free PMC article.
-
Childhood Trauma, the HPA Axis and Psychiatric Illnesses: A Targeted Literature Synthesis.Front Psychiatry. 2022 May 6;13:748372. doi: 10.3389/fpsyt.2022.748372. eCollection 2022. Front Psychiatry. 2022. PMID: 35599780 Free PMC article. Review.
-
Two Chronic Stress Models Based on Movement Restriction in Rats Respond Selectively to Antidepressant Drugs: Aldolase C As a Potential Biomarker.Int J Neuropsychopharmacol. 2015 Mar 26;18(10):pyv038. doi: 10.1093/ijnp/pyv038. Int J Neuropsychopharmacol. 2015. PMID: 25813018 Free PMC article.
References
-
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behavioural Brain Research. 1996;78(1):57–65. - PubMed
-
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25(6):295–301. - PubMed
-
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature Reviews Neuroscience. 2006;7(9):697–709. - PubMed
-
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Research Reviews. 2005;50(1):126–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

