Renal endothelial dysfunction in diabetic nephropathy
- PMID: 24720460
- PMCID: PMC4657140
- DOI: 10.2174/1871529x14666140401110841
Renal endothelial dysfunction in diabetic nephropathy
Abstract
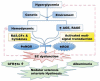
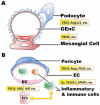
Endothelial dysfunction has been posited to play an important role in the pathogenesis of diabetic nephropathy (DN). Due to the heterogeneity of endothelial cells (ECs), it is difficult to generalize about endothelial responses to diabetic stimuli. At present, there are limited techniques fordirectly measuring EC function in vivo, so diagnosis of endothelial disorders still largely depends on indirect assessment of mediators arising from EC injury. In the kidney microcirculation, both afferent and efferent arteries, arterioles and glomerular endothelial cells (GEnC) have all been implicated as targets of diabetic injury. Both hyperglycemia per se, as well as the metabolic consequences of glucose dysregulation, are thought to lead to endothelial cell dysfunction. In this regard, endothelial nitric oxide synthase (eNOS) plays a central role in EC dysfunction. Impaired eNOS activity can occur at numerous levels, including enzyme uncoupling, post-translational modifications, internalization and decreased expression. Reduced nitric oxide (NO) bioavailability exacerbates oxidative stress, further promoting endothelial dysfunction and injury. The injured ECs may then function as active signal transducers of metabolic, hemodynamic and inflammatory factors that modify the function and morphology of the vessel wall and interact with adjacent cells, which may activate a cascade of inflammatory and proliferative and profibrotic responses in progressive DN. Both pharmacological approaches and potential regenerative therapies hold promise for restoration of impaired endothelial cells in diabetic nephropathy.
Figures
References
-
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902–16. - PubMed
-
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. - PubMed
-
- Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp. Biol. Med. (Maywood) 2008;233(1):4–11. - PubMed
-
- Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr. Rev. 2001;22(1):36–52. - PubMed
-
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001;88(2):E14–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK51265/DK/NIDDK NIH HHS/United States
- DK79341/DK/NIDDK NIH HHS/United States
- P01 DK038226/DK/NIDDK NIH HHS/United States
- R01 DK095785/DK/NIDDK NIH HHS/United States
- R01 DK051265/DK/NIDDK NIH HHS/United States
- DK38226/DK/NIDDK NIH HHS/United States
- DK62794/DK/NIDDK NIH HHS/United States
- R01 DK062794/DK/NIDDK NIH HHS/United States
- I01 BX000320/BX/BLRD VA/United States
- P30 DK079341/DK/NIDDK NIH HHS/United States
- DK95785/DK/NIDDK NIH HHS/United States
- VA999999/ImVA/Intramural VA/United States
- R24 DK103067/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

