Congested C-C bonds by Pd-catalyzed enantioselective allyl-allyl cross-coupling, a mechanism-guided solution
- PMID: 24720611
- PMCID: PMC4211291
- DOI: 10.1021/ja502280w
Congested C-C bonds by Pd-catalyzed enantioselective allyl-allyl cross-coupling, a mechanism-guided solution
Abstract
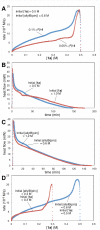
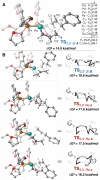
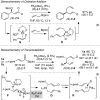
Under the influence of a chiral bidentate diphosphine ligand, the Pd-catalyzed asymmetric cross-coupling of allylboron reagents and allylic electrophiles establishes 1,5-dienes with adjacent stereocenters in high regio- and stereoselectivity. A mechanistic study of the coupling utilizing reaction calorimetry and density functional theory analysis suggests that the reaction operates through an inner-sphere 3,3'-reductive elimination pathway, which is both rate-defining and stereodefining. Coupled with optimized reaction conditions, this mechanistic detail is used to expand the scope of allyl-allyl couplings to allow the generation of 1,5-dienes with tertiary centers adjacent to quaternary centers as well as a unique set of cyclic structures.
Figures

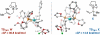
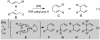

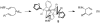

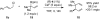
Similar articles
-
Enantioselective Coupling of Dienes and Phosphine Oxides.J Am Chem Soc. 2018 Dec 5;140(48):16450-16454. doi: 10.1021/jacs.8b11150. Epub 2018 Nov 19. J Am Chem Soc. 2018. PMID: 30451496 Free PMC article.
-
Construction of vicinal tertiary and all-carbon quaternary stereocenters via Ir-catalyzed regio-, diastereo-, and enantioselective allylic alkylation and applications in sequential Pd catalysis.J Am Chem Soc. 2013 Jul 24;135(29):10626-9. doi: 10.1021/ja4052075. Epub 2013 Jul 12. J Am Chem Soc. 2013. PMID: 23829704 Free PMC article.
-
Pd-catalyzed enantioselective allyl-allyl cross-coupling.J Am Chem Soc. 2010 Aug 11;132(31):10686-8. doi: 10.1021/ja105161f. J Am Chem Soc. 2010. PMID: 20681700 Free PMC article.
-
Palladium-catalyzed chemoselective allylic substitution, Suzuki-Miyaura cross-coupling, and allene formation of bifunctional 2-B(pin)-substituted allylic acetate derivatives.Chemistry. 2014 Sep 8;20(37):11726-39. doi: 10.1002/chem.201402353. Epub 2014 Jul 30. Chemistry. 2014. PMID: 25077980 Free PMC article.
-
The Heck reaction applied to 1,3- and 1,2-unsaturated derivatives, a way towards molecular complexity.Molecules. 2010 Apr 13;15(4):2667-85. doi: 10.3390/molecules15042667. Molecules. 2010. PMID: 20428072 Free PMC article. Review.
Cited by
-
Heck Reaction of Electronically Diverse Tertiary Alkyl Halides.Org Lett. 2018 Jan 19;20(2):357-360. doi: 10.1021/acs.orglett.7b03591. Epub 2018 Jan 5. Org Lett. 2018. PMID: 29303271 Free PMC article.
-
A Cascade Reaction of Cinnamyl Azides with Acrylates Directly Generates Tetrahydro-Pyrrolo-Pyrazole Heterocycles.J Org Chem. 2020 May 1;85(9):6044-6059. doi: 10.1021/acs.joc.0c00535. Epub 2020 Apr 20. J Org Chem. 2020. PMID: 32281795 Free PMC article.
-
Additive-Free Pd-Catalyzed α-Allylation of Imine-Containing Heterocycles.Org Lett. 2017 Jan 6;19(1):126-129. doi: 10.1021/acs.orglett.6b03407. Epub 2016 Dec 12. Org Lett. 2017. PMID: 27936786 Free PMC article.
-
Borylated Cyclopropanes as Spring-Loaded Entities: Access to Vicinal Tertiary and Quaternary Carbon Stereocenters in Acyclic Systems.J Am Chem Soc. 2022 Sep 14;144(36):16298-16302. doi: 10.1021/jacs.2c07394. Epub 2022 Aug 30. J Am Chem Soc. 2022. PMID: 36041738 Free PMC article.
-
Synthesis and Functionalization of Tertiary Propargylic Boronic Esters by Alkynyllithium-Mediated 1,2-Metalate Rearrangement of Borylated Cyclopropanes.Org Lett. 2022 Dec 9;24(48):8901-8906. doi: 10.1021/acs.orglett.2c03756. Epub 2022 Nov 29. Org Lett. 2022. PMID: 36446049 Free PMC article.
References
-
- Kazmaier U, editor. Transition Metal Catalyzed Enantioselective Allylic Substitutions in Organic Synthesis. Vol. 38. Springer: Heidel-berg: 2012. For a general review see. Topics in Organometallic Chemistry.
- Trost BM, Van Vranken DL. Chem. Rev. 1996;96:395. For reviews regarding prochiral allyl electrophiles, see. - PubMed
- Helmchen G. J. Organometallic Chem. 1999;576:203.
-
- Negishi E-i., Liao B. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E-i, de Meijere A., editors. Vol. 1. Wiley-Interscience; West Lafayette: 2002. pp. 591–596.
-
- Trost BM, Keinan E. Tetrahedron Lett. 1980;21:2595. For nonselective or linear selective allyl-allyl couplings, see:
- Godschalx J, Stille JK. Tetrahedron Lett. 1980;21:2599.
- van Heerden FR, Huyser JJ, Williams DBG, Holzapfel CW. Tetrahedron Lett. 1998;39:5281.
- Nakamura H, Bao M, Yamamoto Y. Angew. Chem. Int. Ed. 2001;40:3208. - PubMed
- Flegeau EF, Schneider U, Kobayashi S. Chem. –Eur. J. 2009;15:12247. - PubMed
- Jiménez-Aquino A, Flegeau EF, Schneider U, Kobayashi S. Chem. Commun. 2011;47:9456. - PubMed
- Trost BM, Pietrusiewicz KM. Tetrahedron Lett. 1985;26:4039. For intramolecular allyl-allyl couplings, see.
- Cuerva JM, Gómez,-Bengoa E, Méndez M, Echavarren AM. J. Org. Chem. 1997;62:7540.
- Keinan E, Peretz M. J. Org. Chem. 1983;48:5302. For related couplings, see:
- Keinan E, Bosch E. J. Org. Chem. 1986;51:4006.
-
- Goliaszewski A, Schwartz J. J. Am. Chem. Soc. 1984;106:5028. For mechanistic studies on allyl-allyl couplings, see:
- Goliaszewski A, Schwartz J. Tetrahedron. 1985;41:5779.
- Goliaszewski A, Schwartz J. Organometallics. 1985;4:417.
- Jolly P. Angew. Chem. Int. Ed. Eng. 1985;24:283.
- Kraus J, Bonrath W, Pörschke KR. Organometallics. 1992;11:1158.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

