Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer
- PMID: 24720900
- PMCID: PMC3997215
- DOI: 10.1186/1471-2407-14-251
Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer
Abstract
Background: Renal cell carcinoma (RCC) is a malignant disease that demonstrates resistance to standard chemotherapeutic agents. Yet Active immunization using genetically modified dendritic cells holds promise for the adjuvant treatment of malignancies to eradicate or control residual disease. Cytokine-induced killer (CIK) cells are a heterogeneous population of effector CD8+ T cells with diverse TCR specificities, possessing non-MHC-restricted cytolytic activities against tumor cells. Clinical studies have confirmed benefit and safety of CIK cell-based therapy for patients with malignancies. This clinical trial was conducted to evaluate efficacy and safety of genetically modified dendritic cells in combination with Cytokine-Induced Killer Cell (gmDCs-CIK) treatment of patients with RCC.
Methods: 28 patients with advanced renal cancer were admitted to Affiliated Hospital of Academy of Military Medical Sciences from December 2010 to March 2012 and treated by gmDCs-CIK. Clinical efficacy and safety between pre- and post-treatment were compared.
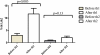
Results: This analysis showed an objective response rate (ORR) of 39% and a disease control rate (DCR) of as 75%. There is no significant relationship between clinical efficacy and whether metastasis occurred or not (P > 0.05). There is no significant relationship between ORR and cycles of treatment (P > 0.05), but DCR was significantly related with cycles of treatment (P < 0.05). No clinically significant side effects were observed. There were no significant changes of T cell subsets including CD3+, CD4+, CD8+, CD4+ CD25+ Treg cells except Th1 in peripheral blood between day 30 after immunotherapy and 1 day before immunotherapy in 11 patients.
Conclusion: DC-CIK is feasible and effective in treating advanced renal cancer and thus provides a new approach.
Trial registration: ClinicalTrials.gov Identifier: NCT01924156. Registration date: August 14, 2013.
Figures
Similar articles
-
A randomized controlled trial of postoperative tumor lysate-pulsed dendritic cells and cytokine-induced killer cells immunotherapy in patients with localized and locally advanced renal cell carcinoma.Chin Med J (Engl). 2012 Nov;125(21):3771-7. Chin Med J (Engl). 2012. PMID: 23106871 Clinical Trial.
-
[Efficacy of autologous renal tumor cell lysate-loaded dendritic cell vaccine in combination with cytokine-induced killer cells on advanced renal cell carcinoma--a report of ten cases].Ai Zheng. 2006 May;25(5):625-30. Ai Zheng. 2006. PMID: 16687087 Chinese.
-
Retrospective analysis on the efficacy of sunitinib/sorafenib in combination with dendritic cells-cytokine-induced killer in metastasis renal cell carcinoma after radical nephrectomy.J Cancer Res Ther. 2018 Jun;14(Supplement):S427-S432. doi: 10.4103/0973-1482.180609. J Cancer Res Ther. 2018. PMID: 29970701
-
Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell /dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: A systematic review and meta-analysis.Cytotherapy. 2016 Sep;18(9):1162-77. doi: 10.1016/j.jcyt.2016.05.015. Epub 2016 Jul 12. Cytotherapy. 2016. PMID: 27421742
-
Cytokine-induced killer cells/dendritic cells and cytokine-induced killer cells immunotherapy for the treatment of esophageal cancer: A meta-analysis.Medicine (Baltimore). 2021 Apr 2;100(13):e24519. doi: 10.1097/MD.0000000000024519. Medicine (Baltimore). 2021. PMID: 33787569 Free PMC article.
Cited by
-
Adjuvant cellular immunotherapy in patients with resected primary non-small cell lung cancer.Oncoimmunology. 2015 May 27;4(9):e1038017. doi: 10.1080/2162402X.2015.1038017. eCollection 2015 Sep. Oncoimmunology. 2015. PMID: 26405607 Free PMC article.
-
NaHCO3 enhances the antitumor activities of cytokine-induced killer cells against hepatocellular carcinoma HepG2 cells.Oncol Lett. 2016 Nov;12(5):3167-3174. doi: 10.3892/ol.2016.5112. Epub 2016 Sep 9. Oncol Lett. 2016. PMID: 27899977 Free PMC article.
-
Feasibility study of DCs/CIKs combined with thoracic radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer.Radiat Oncol. 2016 Apr 21;11:60. doi: 10.1186/s13014-016-0635-5. Radiat Oncol. 2016. PMID: 27097970 Free PMC article. Clinical Trial.
-
Promise of cancer stem cell vaccine.Hum Vaccin Immunother. 2015;11(12):2796-9. doi: 10.1080/21645515.2015.1083661. Hum Vaccin Immunother. 2015. PMID: 26337078 Free PMC article.
-
Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment.J Cancer Res Clin Oncol. 2017 Dec;143(12):2607-2618. doi: 10.1007/s00432-017-2513-4. Epub 2017 Sep 4. J Cancer Res Clin Oncol. 2017. PMID: 28871458 Free PMC article. Clinical Trial.
References
-
- Escudier B. Emerging immunotherapies for renal cell carcinoma. Ann Oncol. 2012;8:35–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

