Combining motivational and volitional strategies to promote unsupervised walking in patients with fibromyalgia: study protocol for a randomized controlled trial
- PMID: 24721143
- PMCID: PMC4026054
- DOI: 10.1186/1745-6215-15-120
Combining motivational and volitional strategies to promote unsupervised walking in patients with fibromyalgia: study protocol for a randomized controlled trial
Abstract
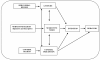
Background: Fibromyalgia patients are often advised to engage in regular low- to moderate-intensity physical exercise. The need of fibromyalgia patients to walk has been stressed in previous research. Behavioral self-regulation theories suggest that a combination of motivational aspects (to develop or strengthen a behavioral intention: Theory of Planned Behavior) and volitional aspects (engagement of intention in behavior: implementation intentions) is more effective than a single intervention. In this paper, we describe a protocol for identifying the motivational processes (using the Theory of Planned Behavior) involved in the practice of walking (phase I) and for studying the efficacy of an intervention that combines motivational and volitional contents to enhance the acquisition and continuation of this exercise behavior (phase II). The paper also shows the characteristics of eligible individuals (women who do not walk) and ineligible populations (women who walk or do not walk because of comorbidity without medical recommendation to walk). Both groups consist of members of any of four patients' associations in Spain who are between 18 and 70 years of age and meet the London Fibromyalgia Epidemiology Study Screening Questionnaire criteria for fibromyalgia. Furthermore, using this study protocol, we will explore the characteristics of participants (eligible women who agreed to participate in the study) and nonparticipants (eligible women who refused to participate).
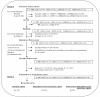
Methods/design: Two studies will be conducted: Phase I will be a cross-sectional study, and phase II will be a triple-blind, randomized longitudinal study with two treatment groups and one active control group. The questionnaires were sent to a total of 2,227 members of four patients' associations in Spain. A total of 920 participants with fibromyalgia returned the questionnaires, and 582 were ultimately selected to participate.
Discussion: The first data gathered have allowed us to identify the characteristics of the study population and they support the appropriateness of the inclusion criteria.. When the study is complete, the results will enable us to establish whether this kind of intervention can be used as a self-regulation tool for increasing and maintaining walking as unsupervised physical exercise of low to moderate intensity in fibromyalgia patients.
Trial registration number: ISRCTN68584893.
Figures

References
-
- Busch AJ, Barber KAR, Overend TJ, Peloso PMJ, Schachter CL. The Cochrane Library, Issue 4. Oxford: Update Software; Exercise for treating fibromyalgia syndrome (Cochrane Review) Available at: http://www.update-software.com (translated from The Cochrane Library, Issue 3. Chichester, UK: John Wiley & Sons; 8 October 2008)
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

