Subclinical inflammation on MRI of hand and foot of anticitrullinated peptide antibody-negative arthralgia patients at risk for rheumatoid arthritis
- PMID: 24721160
- PMCID: PMC4060237
- DOI: 10.1186/ar4536
Subclinical inflammation on MRI of hand and foot of anticitrullinated peptide antibody-negative arthralgia patients at risk for rheumatoid arthritis
Abstract
Introduction: It is known that anticitrullinated peptide antibody (ACPA)-positive rheumatoid arthritis (RA) has a preclinical phase. Whether this phase is also present in ACPA-negative RA is unknown. To determine this, we studied ACPA-negative arthralgia patients who were considered prone to progress to RA for local subclinical inflammation observed on hand and foot magnetic resonance imaging (MRI) scans.
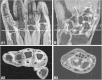
Methods: We studied a total of 64 ACPA-negative patients without clinically detectable arthritis and with arthralgia of the small joints within the previous 1 year. Because of the character of the patients' symptoms, the rheumatologists considered these patients to be prone to progress to RA. For comparisons, we evaluated 19 healthy, symptom-free controls and 20 ACPA-negative RA patients, who were identified according to the 1987 American Rheumatism Association criteria. All participants underwent MRI of unilateral wrist, metacarpophalangeal and metatarsophalangeal joints. Synovitis and bone marrow oedema (BME) were scored according to the OMERACT rheumatoid arthritis magnetic resonance imaging scoring system, and the scores were summed to yield the 'MRI inflammation score'. Scores were compared between groups. Among the ACPA-negative arthralgia patients, MRI inflammation scores were related to C-reactive protein (CRP) levels and the tenderness of scanned joints.
Results: MRI inflammation scores increased progressively among the groups of controls and ACPA-negative arthralgia and RA patients (median scores = 0, 1 and 10, respectively; P < 0.001). The MRI inflammation scores of ACPA-negative arthralgia patients were significantly higher than those of controls (P = 0.018). In particular, the synovitis scores were higher in ACPA-negative arthralgia patients (P = 0.046). Among the ACPA-negative arthralgia patients, inflammation was observed predominantly in the wrist (53%). The synovitis scores were associated with CRP levels (P = 0.007) and joint tenderness (P = 0.026). Despite the limited follow-up duration, five patients developed clinically detectable arthritis. These five patients had higher scores for MRI inflammation (P = 0.001), synovitis (P = 0.002) and BME (P = 0.003) compared to the other patients.
Conclusion: Subclinical synovitis was observed in the small joints of ACPA-negative arthralgia patients, and especially in patients whose conditions progressed to clinically detectable arthritis. This finding suggests the presence of a preclinical phase in ACPA-negative RA. Further longitudinal studies of these lesions and patients are required to confirm this hypothesis.
Figures

Similar articles
-
MRI of hand and foot joints of patients with anticitrullinated peptide antibody positive arthralgia without clinical arthritis.Ann Rheum Dis. 2013 Sep 1;72(9):1540-4. doi: 10.1136/annrheumdis-2012-202628. Epub 2013 Jan 19. Ann Rheum Dis. 2013. PMID: 23334211
-
Are rheumatoid arthritis patients discernible from other early arthritis patients using 1.5T extremity magnetic resonance imaging? a large cross-sectional study.J Rheumatol. 2014 Aug;41(8):1630-7. doi: 10.3899/jrheum.131169. Epub 2014 Jul 15. J Rheumatol. 2014. PMID: 25028382
-
Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis.Ann Rheum Dis. 2016 Oct;75(10):1824-30. doi: 10.1136/annrheumdis-2015-208138. Epub 2015 Nov 27. Ann Rheum Dis. 2016. PMID: 26613769
-
OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system.J Rheumatol. 2003 Jun;30(6):1385-6. J Rheumatol. 2003. PMID: 12784422 Review.
-
The value of MRI for detecting subclinical joint inflammation in clinically suspect arthralgia.RMD Open. 2022 Jul;8(2):e002128. doi: 10.1136/rmdopen-2021-002128. RMD Open. 2022. PMID: 35820736 Free PMC article. Review.
Cited by
-
MSC-microvesicles protect cartilage from degradation in early rheumatoid arthritis via immunoregulation.J Nanobiotechnology. 2024 Nov 4;22(1):673. doi: 10.1186/s12951-024-02922-6. J Nanobiotechnology. 2024. PMID: 39497131 Free PMC article.
-
EULAR points to consider for conducting clinical trials and observational studies in individuals at risk of rheumatoid arthritis.Ann Rheum Dis. 2021 Oct;80(10):1286-1298. doi: 10.1136/annrheumdis-2021-220884. Epub 2021 Aug 6. Ann Rheum Dis. 2021. PMID: 34362746 Free PMC article.
-
Application of Ultrasound in Assessment of Biologics Efficacy in Patients with Rheumatoid Arthritis.J Med Ultrasound. 2022 Jan 6;30(2):79-80. doi: 10.4103/JMU.JMU_147_21. eCollection 2022 Apr-Jun. J Med Ultrasound. 2022. PMID: 35832360 Free PMC article. No abstract available.
-
Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start?Semin Immunopathol. 2017 Jun;39(4):423-435. doi: 10.1007/s00281-017-0620-6. Epub 2017 Mar 23. Semin Immunopathol. 2017. PMID: 28337522 Free PMC article. Review.
-
Is joint pain in patients with arthralgia suspicious for progression to rheumatoid arthritis explained by subclinical inflammation? A cross-sectional MRI study.Rheumatology (Oxford). 2019 Jan 1;58(1):86-93. doi: 10.1093/rheumatology/key220. Rheumatology (Oxford). 2019. PMID: 30137540 Free PMC article.
References
-
- Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. - DOI - PubMed
-
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

