In vivo evidence for mTORC2-mediated actin cytoskeleton rearrangement in neurons
- PMID: 24721730
- PMCID: PMC4201605
- DOI: 10.4161/bioa.26497
In vivo evidence for mTORC2-mediated actin cytoskeleton rearrangement in neurons
Abstract
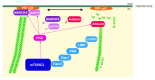
The mammalian target of rapamycin (mTOR) assembles into two distinct multi-protein complexes called mTORC1 and mTORC2. While mTORC1 controls the signaling pathways important for cell growth, the physiological function of mTORC2 is only partially known. Here we comment on recent work on gene-targeted mice lacking mTORC2 in the cerebellum or the hippocampus that provided strong evidence that mTORC2 plays an important role in neuron morphology and synapse function. We discuss that this phenotype might be based on the perturbed regulation of the actin cytoskeleton and the lack of activation of several PKC isoforms. The fact that PKC isoforms and their targets have been implicated in neurological disease including spinocerebellar ataxia and that they have been shown to affect learning and memory, suggests that aberration of mTORC2 signaling might be involved in diseases of the brain.
Keywords: Adducin; GAP-43; MARCKS; PKC; Purkinje cell; Rac1; Tiam1; dendrite; rictor; synaptic plasticity.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
