Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study
- PMID: 24721888
- PMCID: PMC4011437
- DOI: 10.2215/CJN.02650313
Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study
Abstract
Background and objectives: The two largest studies of mammalian target of rapamycin inhibitor treatment of autosomal dominant polycystic kidney disease (ADPKD) demonstrated no clear benefit on the primary endpoint of total kidney volume (TKV) or on eGFR. The present study evaluated two levels of rapamycin on the 12-month change in (125)I-iothalamate GFR (iGFR) as the primary endpoint and TKV secondarily.
Design, setting, participants, & measurements: In a 12-month open-label pilot study, 30 adult patients with ADPKD were randomly assigned to low-dose (LD) rapamycin (rapamycin trough blood level, 2-5 ng/ml) (LD group, n=10), standard-dose (STD) rapamycin trough level (>5-8 ng/ml) (STD group, n=10), or standard care (SC group, n=10). They were evaluated with iGFR and noncontrast computed tomography.
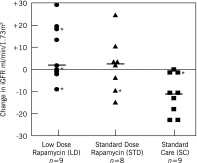
Results: Change in iGFR at 12 months was significantly higher in the LD group (7.7±12.5 ml/min per 1.73 m(2); n=9) than in the SC group (-11.2 ± 9.1 ml/min per 1.73 m(2); n=9) (LD versus SC: P<0.01). Change in iGFR at 12 months in the STD group (1.6 ± 12.1 ml/min per 1.73 m(2); n=8) was not significantly greater than that in the SC group (P=0.07), but it was in the combined treatment groups (LD+STD versus SC: P<0.01). Neither eGFR calculated by the CKD-Epidemiology Collaboration equation nor TKV (secondary endpoint) changed significantly from baseline to 12 months in any of the groups. On the basis of results of the mixed model, during the study, patients in the LD group had significantly lower trough blood levels of rapamycin (mean range ± SD, 2.40 ± 0.64 to 2.90 ± 1.20 ng/ml) compared with those in the STD group (3.93 ± 2.27 to 5.77 ± 1.06 ng/ml) (P<0.01).
Conclusion: Patients with ADPKD receiving LD rapamycin demonstrated a significant increase in iGFR compared with those receiving standard care, without a significant effect on TKV after 12 months.
Keywords: ADPKD; iothalamate GFR; rapamycin.
Figures
Comment in
- 831–836
Similar articles
-
Effect of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease by CKD Stage: Results from the TEMPO 3:4 Trial.Clin J Am Soc Nephrol. 2016 May 6;11(5):803-811. doi: 10.2215/CJN.06300615. Epub 2016 Feb 23. Clin J Am Soc Nephrol. 2016. PMID: 26912543 Free PMC article. Clinical Trial.
-
Efficacy and safety of mTOR inhibitor therapy in patients with early-stage autosomal dominant polycystic kidney disease: a meta-analysis of randomized controlled trials.Am J Med Sci. 2012 Dec;344(6):491-7. doi: 10.1097/MAJ.0b013e318256754f. Am J Med Sci. 2012. PMID: 22902868 Review.
-
Effect of Sirolimus on Disease Progression in Patients with Autosomal Dominant Polycystic Kidney Disease and CKD Stages 3b-4.Clin J Am Soc Nephrol. 2016 May 6;11(5):785-794. doi: 10.2215/CJN.09900915. Epub 2016 Feb 22. Clin J Am Soc Nephrol. 2016. PMID: 26912555 Free PMC article. Clinical Trial.
-
Effect of Sirolimus on Native Total Kidney Volume After Transplantation in Patients with Autosomal Dominant Polycystic Kidney Disease: A Randomized Controlled Pilot Study.Transplant Proc. 2018 Jun;50(5):1243-1248. doi: 10.1016/j.transproceed.2018.02.060. Transplant Proc. 2018. PMID: 29880342 Clinical Trial.
-
Long-term treatment with mammalian target of rapamycin inhibitor does not benefit patients with autosomal dominant polycystic kidney disease: a meta-analysis.Nephron Clin Pract. 2013;124(1-2):10-6. doi: 10.1159/000354398. Epub 2013 Sep 6. Nephron Clin Pract. 2013. PMID: 24022660 Review.
Cited by
-
Folate conjugated nanomedicines for selective inhibition of mTOR signaling in polycystic kidneys at clinically relevant doses.Biomaterials. 2023 Nov;302:122329. doi: 10.1016/j.biomaterials.2023.122329. Epub 2023 Sep 13. Biomaterials. 2023. PMID: 37722182 Free PMC article.
-
Interventions for preventing the progression of autosomal dominant polycystic kidney disease.Cochrane Database Syst Rev. 2024 Oct 2;10(10):CD010294. doi: 10.1002/14651858.CD010294.pub3. Cochrane Database Syst Rev. 2024. PMID: 39356039
-
Roles of mTOR complexes in the kidney: implications for renal disease and transplantation.Nat Rev Nephrol. 2016 Oct;12(10):587-609. doi: 10.1038/nrneph.2016.108. Epub 2016 Aug 1. Nat Rev Nephrol. 2016. PMID: 27477490 Free PMC article. Review.
-
Lowe syndrome patient cells display mTOR- and RhoGTPase-dependent phenotypes alleviated by rapamycin and statins.Hum Mol Genet. 2020 Jun 27;29(10):1700-1715. doi: 10.1093/hmg/ddaa086. Hum Mol Genet. 2020. PMID: 32391547 Free PMC article.
-
Use of mammalian target of rapamycin inhibitors in patient with autosomal dominant polycystic kidney disease: an updated meta-analysis.Int Urol Nephrol. 2019 Nov;51(11):2015-2025. doi: 10.1007/s11255-019-02292-1. Epub 2019 Oct 1. Int Urol Nephrol. 2019. PMID: 31578673
References
-
- Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 - PubMed
-
- King BF, Reed JE, Bergstralh EJ, Sheedy PF, 2nd, Torres VE: Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 11: 1505–1511, 2000 - PubMed
-
- Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 - PubMed
-
- Bae KT, Grantham JJ: Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 96–106, 2010 - PubMed
-
- Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

