Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease
- PMID: 24721893
- PMCID: PMC4011448
- DOI: 10.2215/CJN.08350813
Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease
Abstract
Background and objectives: In autosomal dominant polycystic kidney disease (ADPKD), progressive kidney cyst formation commonly leads to ESRD. Because important manifestations of ADPKD may be evident in childhood, early intervention may have the largest effect on long-term outcome. Statins are known to slow progressive nephropathy in animal models of ADPKD. This randomized double-blind placebo-controlled phase III clinical trial was conducted from 2007 to 2012 to assess the effect of pravastatin on height-corrected total kidney volume (HtTKV) and left ventricular mass index (LVMI) by magnetic resonance imaging (MRI) and urine microalbumin excretion (UAE) in children and young adults with ADPKD.
Designs, setting, participants, & measurements: There were 110 pediatric participants with ADPKD and normal kidney function receiving lisinopril who were randomized to treatment with pravastatin or placebo for a 3-year period with evaluation at 0, 18, and 36 months. The primary outcome variable was a ≥ 20% change in HtTKV, LVMI, or UAE over the study period.
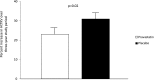
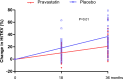
Results: Ninety-one participants completed the 3-year study (83%). Fewer participants receiving pravastatin achieved the primary endpoint compared with participants receiving placebo (69% versus 88%; P=0.03). This was due primarily to a lower proportion reaching the increase in HtTKV (46% versus 68%; P=0.03), with similar findings observed between study groups for LVMI (25% versus 38%; P=0.18) and UAE (47% versus 39%; P=0.50). The percent change in HtTKV adjusted for age, sex, and hypertension status over the 3-year period was significantly decreased with pravastatin (23% ± 3% versus 31% ± 3%; P=0.02).
Conclusions: Pravastatin is an effective agent to slow progression of structural kidney disease in children and young adults with ADPKD. These findings support a role for early intervention with pravastatin in this condition.
Keywords: children; kidney volume; polycystic kidney disease; statins.
Figures

Comment in
- 831–836 doi: 10.2215/CJN.02480314
References
-
- Somlo S, Chapman AB: Autosomal dominant polycystic kidney disease. In: Schrier's Diseases of the Kidney, edited by Coffman TM, Falk RJ, Molitoris BA, Neilson EG, Schrier RW, 9th Ed., Philadelphia, Lippincott Williams & Wilkins, 2012, pp 519–563
-
- Pretorius DH, Lee ME, Manco-Johnson ML, Weingast GR, Sedman AB, Gabow PA: Diagnosis of autosomal dominant polycystic kidney disease in utero and in the young infant. J Ultrasound Med 6: 249–255, 1987 - PubMed
-
- Hafez KS, Inman SR, Stowe NT, Novick AC: Renal hemodynamic effects of lovastatin in a renal ablation model. Urology 48: 862–867, 1996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

