Unexpected role of the steroid-deficiency protein ecdysoneless in pre-mRNA splicing
- PMID: 24722212
- PMCID: PMC3983036
- DOI: 10.1371/journal.pgen.1004287
Unexpected role of the steroid-deficiency protein ecdysoneless in pre-mRNA splicing
Abstract
The steroid hormone ecdysone coordinates insect growth and development, directing the major postembryonic transition of forms, metamorphosis. The steroid-deficient ecdysoneless1 (ecd1) strain of Drosophila melanogaster has long served to assess the impact of ecdysone on gene regulation, morphogenesis, or reproduction. However, ecd also exerts cell-autonomous effects independently of the hormone, and mammalian Ecd homologs have been implicated in cell cycle regulation and cancer. Why the Drosophila ecd1 mutants lack ecdysone has not been resolved. Here, we show that in Drosophila cells, Ecd directly interacts with core components of the U5 snRNP spliceosomal complex, including the conserved Prp8 protein. In accord with a function in pre-mRNA splicing, Ecd and Prp8 are cell-autonomously required for survival of proliferating cells within the larval imaginal discs. In the steroidogenic prothoracic gland, loss of Ecd or Prp8 prevents splicing of a large intron from CYP307A2/spookier (spok) pre-mRNA, thus eliminating this essential ecdysone-biosynthetic enzyme and blocking the entry to metamorphosis. Human Ecd (hEcd) can substitute for its missing fly ortholog. When expressed in the Ecd-deficient prothoracic gland, hEcd re-establishes spok pre-mRNA splicing and protein expression, restoring ecdysone synthesis and normal development. Our work identifies Ecd as a novel pre-mRNA splicing factor whose function has been conserved in its human counterpart. Whether the role of mammalian Ecd in cancer involves pre-mRNA splicing remains to be discovered.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
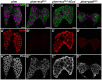
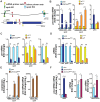
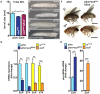
References
-
- Thummel CS (2001) Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell 1: 453–465. - PubMed
-
- Mirth CK, Riddiford LM (2007) Size assessment and growth control: how adult size is determined in insects. Bioessays 29: 344–355. - PubMed
-
- Ou Q, King-Jones K (2013) What goes up must come down: transcription factors have their say in making ecdysone pulses. Curr Top Dev Biol 103: 35–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

