Tetraphenylporphyrin derivative specifically blocks members of the voltage-gated potassium channel subfamily Kv1
- PMID: 24722265
- PMCID: PMC4042482
- DOI: 10.4161/chan.25848
Tetraphenylporphyrin derivative specifically blocks members of the voltage-gated potassium channel subfamily Kv1
Abstract
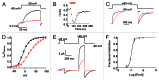
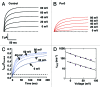
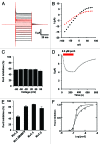
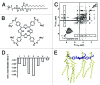
Tetraphenylporphyrin derivatives represent a promising class of high-affinity ligands for voltage-gated potassium (Kv) channels. Herein, we investigated the mode of Kv channel block of one tetraphenylporphyrin derivative, por3, using electrophysiological methods, structure-based mutagenesis, and solid-state NMR spectroscopy. The combined data showed that por3 specifically blocks Kv1.x channels. Unexpectedly, 2 different por3 binding modes lead to Kv1.x channel block exerted through multiple por3 binding sites: first, por3 interacts in a highly cooperative and specific manner with the voltage sensor domain stabilizing closed Kv1 channel state(s). Therefore, stronger depolarization is needed to activate Kv1.x channels in the presence of por3. Second, por3 bind to a single site at the external pore entrance to block the ion conduction pathway of activated Kv1.x channels. This block is voltage-independent. Por3 appears to have equal affinities for voltage-sensor and pore. However, at negative voltage and low por3 concentration, por3 gating modifier properties prevail due to the high cooperativity of binding. By contrast, at positive voltages, when Kv1.x channels are fully activated, por3 pore blocking properties predominate.
Keywords: Voltage-gated potassium-channel; gating modifier; liposomes; porphyrin; solid-state NMR.
Figures


Similar articles
-
A novel spider toxin as a selective antagonist of the Kv1 subfamily of voltage-gated potassium channels through gating modulation.J Biol Chem. 2025 Apr;301(4):108341. doi: 10.1016/j.jbc.2025.108341. Epub 2025 Feb 22. J Biol Chem. 2025. PMID: 39993525 Free PMC article.
-
Studies of Conorfamide-Sr3 on Human Voltage-Gated Kv1 Potassium Channel Subtypes.Mar Drugs. 2020 Aug 13;18(8):425. doi: 10.3390/md18080425. Mar Drugs. 2020. PMID: 32823677 Free PMC article.
-
Complexes of Peptide Blockers with Kv1.6 Pore Domain: Molecular Modeling and Studies with KcsA-Kv1.6 Channel.J Neuroimmune Pharmacol. 2017 Jun;12(2):260-276. doi: 10.1007/s11481-016-9710-9. Epub 2016 Sep 17. J Neuroimmune Pharmacol. 2017. PMID: 27640211
-
Chimeras of KcsA and Kv1 as a bioengineering tool to study voltage-gated potassium channels and their ligands.Biochem Pharmacol. 2021 Aug;190:114646. doi: 10.1016/j.bcp.2021.114646. Epub 2021 Jun 4. Biochem Pharmacol. 2021. PMID: 34090876 Review.
-
Marine Toxins Targeting Kv1 Channels: Pharmacological Tools and Therapeutic Scaffolds.Mar Drugs. 2020 Mar 20;18(3):173. doi: 10.3390/md18030173. Mar Drugs. 2020. PMID: 32245015 Free PMC article. Review.
Cited by
-
Water Accessibility Refinement of the Extended Structure of KirBac1.1 in the Closed State.Front Mol Biosci. 2021 Nov 30;8:772855. doi: 10.3389/fmolb.2021.772855. eCollection 2021. Front Mol Biosci. 2021. PMID: 34917650 Free PMC article.
References
-
- Garcia ML, Kaczorowski GJ. Potassium channels as targets for therapeutic intervention. Sci STKE. 2005;2005:pe46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
