Tetraphenylporphyrin derivative specifically blocks members of the voltage-gated potassium channel subfamily Kv1
- PMID: 24722265
- PMCID: PMC4042482
- DOI: 10.4161/chan.25848
Tetraphenylporphyrin derivative specifically blocks members of the voltage-gated potassium channel subfamily Kv1
Abstract
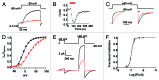
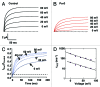
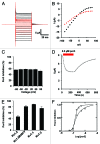
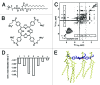
Tetraphenylporphyrin derivatives represent a promising class of high-affinity ligands for voltage-gated potassium (Kv) channels. Herein, we investigated the mode of Kv channel block of one tetraphenylporphyrin derivative, por3, using electrophysiological methods, structure-based mutagenesis, and solid-state NMR spectroscopy. The combined data showed that por3 specifically blocks Kv1.x channels. Unexpectedly, 2 different por3 binding modes lead to Kv1.x channel block exerted through multiple por3 binding sites: first, por3 interacts in a highly cooperative and specific manner with the voltage sensor domain stabilizing closed Kv1 channel state(s). Therefore, stronger depolarization is needed to activate Kv1.x channels in the presence of por3. Second, por3 bind to a single site at the external pore entrance to block the ion conduction pathway of activated Kv1.x channels. This block is voltage-independent. Por3 appears to have equal affinities for voltage-sensor and pore. However, at negative voltage and low por3 concentration, por3 gating modifier properties prevail due to the high cooperativity of binding. By contrast, at positive voltages, when Kv1.x channels are fully activated, por3 pore blocking properties predominate.
Keywords: Voltage-gated potassium-channel; gating modifier; liposomes; porphyrin; solid-state NMR.
Figures


References
-
- Garcia ML, Kaczorowski GJ. Potassium channels as targets for therapeutic intervention. Sci STKE. 2005;2005:pe46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
