The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy
- PMID: 24722445
- PMCID: PMC4005314
- DOI: 10.1681/ASN.2013080830
The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy
Abstract
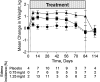
Despite optimal treatment, including renin-angiotensin system (RAS) inhibitors, patients with type 2 diabetic nephropathy have high cardiorenal morbidity and mortality related to residual albuminuria. We evaluated whether or not atrasentan, a selective endothelin A receptor antagonist, further reduces albuminuria when administered concomitantly with maximum tolerated labeled doses of RAS inhibitors. We enrolled 211 patients with type 2 diabetes, urine albumin/creatinine ratios of 300-3500 mg/g, and eGFRs of 30-75 ml/min per 1.73 m(2) in two identically designed, parallel, multinational, double-blind studies. Participants were randomized to placebo (n=50) or to 0.75 mg/d (n=78) or 1.25 mg/d (n=83) atrasentan for 12 weeks. Compared with placebo, 0.75 mg and 1.25 mg atrasentan reduced urine albumin/creatinine ratios by an average of 35% and 38% (95% confidence intervals of 24 to 45 and 28 to 47, respectively) and reduced albuminuria≥30% in 51% and 55% of participants, respectively. eGFR and office BP measurements did not change, whereas 24-hour systolic and diastolic BP, LDL cholesterol, and triglyceride levels decreased significantly in both treatment groups. Use of atrasentan was associated with a significant increase in weight and a reduction in hemoglobin, but rates of peripheral edema, heart failure, or other side effects did not differ between groups. However, more patients treated with 1.25 mg/d atrasentan discontinued due to adverse events. After stopping atrasentan for 30 days, measured parameters returned to pretreatment levels. In conclusion, atrasentan reduced albuminuria and improved BP and lipid spectrum with manageable fluid overload-related adverse events in patients with type 2 diabetic nephropathy receiving RAS inhibitors.
Copyright © 2014 by the American Society of Nephrology.
Figures
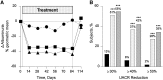
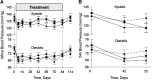
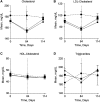
Comment in
- 869–871 doi: 10.1681/ASN.2014020174
References
-
- de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 - PubMed
-
- Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ: Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 45: 281–287, 2005 - PubMed
-
- Cravedi P, Ruggenenti P, Remuzzi G: Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol 8: 301–306, 2012 - PubMed
-
- Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE: Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients with left ventricular hypertrophy and diabetes. J Nephrol 21: 566–569, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

