Alterations in the ubiquitin proteasome system in persistent but not reversible proteinuric diseases
- PMID: 24722446
- PMCID: PMC4214514
- DOI: 10.1681/ASN.2013050522
Alterations in the ubiquitin proteasome system in persistent but not reversible proteinuric diseases
Abstract
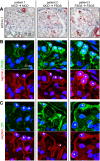
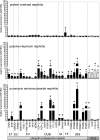
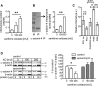
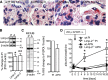
Podocytes are the key cells affected in nephrotic glomerular kidney diseases, and they respond uniformly to injury with cytoskeletal rearrangement. In nephrotic diseases, such as membranous nephropathy and FSGS, persistent injury often leads to irreversible structural damage, whereas in minimal change disease, structural alterations are mostly transient. The factors leading to persistent podocyte injury are currently unknown. Proteolysis is an irreversible process and could trigger persistent podocyte injury through degradation of podocyte-specific proteins. We, therefore, analyzed the expression and functional consequence of the two most prominent proteolytic systems, the ubiquitin proteasome system (UPS) and the autophagosomal/lysosomal system, in persistent and transient podocyte injuries. We show that differential upregulation of both proteolytic systems occurs in persistent human and rodent podocyte injury. The expression of specific UPS proteins in podocytes differentiated children with minimal change disease from children with FSGS and correlated with poor clinical outcome. Degradation of the podocyte-specific protein α-actinin-4 by the UPS depended on oxidative modification in membranous nephropathy. Notably, the UPS was overwhelmed in podocytes during experimental glomerular disease, resulting in abnormal protein accumulation and compensatory upregulation of the autophagosomal/lysosomal system. Accordingly, inhibition of both proteolytic systems enhanced proteinuria in persistent nephrotic disease. This study identifies altered proteolysis as a feature of persistent podocyte injury. In the future, specific UPS proteins may serve as new biomarkers or therapeutic targets in persistent nephrotic syndrome.
Copyright © 2014 by the American Society of Nephrology.
Figures




References
-
- Guan N, Ding J, Zhang J, Yang J: Expression of nephrin, podocin, alpha-actinin, and WT1 in children with nephrotic syndrome. Pediatr Nephrol 18: 1122–1127, 2003 - PubMed
-
- Huh W, Kim DJ, Kim MK, Kim YG, Oh HY, Ruotsalainen V, Tryggvason K: Expression of nephrin in acquired human glomerular disease. Nephrol Dial Transplant 17: 478–484, 2002 - PubMed
-
- Luimula P, Sandström N, Novikov D, Holthöfer H: Podocyte-associated molecules in puromycin aminonucleoside nephrosis of the rat. Lab Invest 82: 713–718, 2002 - PubMed
-
- Ciechanover A, Orian A, Schwartz AL: The ubiquitin-mediated proteolytic pathway: Mode of action and clinical implications. J Cell Biochem Suppl 34: 40–51, 2000 - PubMed
-
- Hochstrasser M: Biochemistry. All in the ubiquitin family. Science 289: 563–564, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

