Human cytomegalovirus inhibits erythropoietin production
- PMID: 24722450
- PMCID: PMC4116070
- DOI: 10.1681/ASN.2013101125
Human cytomegalovirus inhibits erythropoietin production
Abstract
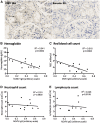
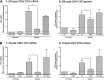
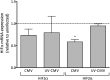
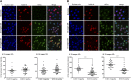
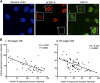
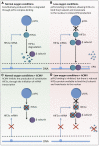
Anemia is a feature of CKD and a complication of renal transplantation, often caused by impaired production of erythropoietin. The kidney is a target organ for human cytomegalovirus (hCMV) in such patients, but it is not known whether hCMV effects erythropoietin production. We found that kidneys from patients with CKD were positive for hCMV protein and that blood levels of hCMV IgG inversely correlated with red blood cell count. In mice, systemic murine cytomegalovirus infection decreased serum erythropoietin levels. In human erythropoietin-producing cells, hCMV inhibited hypoxia-induced expression of erythropoietin mRNA and protein. hCMV early gene expression was responsible, as ultraviolet-inactivated virus had no effect and valganciclovir treatment showed that late gene expression was nonessential. Hypoxia-induced gene transcription is controlled by the transcription factors hypoxia-inducible transcription factor (HIF)-1α and HIF2α, which are constitutively produced but stable only under low oxygen conditions. We found that hCMV inhibited constitutive production of HIF2α mRNA. HIF2α is thought to be the master regulator of erythropoietin transcription. Single-cell analysis revealed that nuclear accumulation of HIF2α was inhibited in hCMV-infected cells, and the extent of inhibition correlated with hCMV protein expression. Our findings suggest that renal hCMV infection could induce or exacerbate anemia in patients.
Copyright © 2014 by the American Society of Nephrology.
Figures


Comment in
-
Cytomegalovirus and anemia: not just for transplant anymore.J Am Soc Nephrol. 2014 Aug;25(8):1613-5. doi: 10.1681/ASN.2014030249. Epub 2014 Apr 10. J Am Soc Nephrol. 2014. PMID: 24722449 Free PMC article. No abstract available.
References
-
- Lorenz M, Kletzmayr J, Perschl A, Furrer A, Hörl WH, Sunder-Plassmann G: Anemia and iron deficiencies among long-term renal transplant recipients. J Am Soc Nephrol 13: 794–797, 2002 - PubMed
-
- Shah N, Al-Khoury S, Afzali B, Covic A, Roche A, Marsh J, Macdougall IC, Goldsmith DJ: Posttransplantation anemia in adult renal allograft recipients: Prevalence and predictors. Transplantation 81: 1112–1118, 2006 - PubMed
-
- Winkelmayer WC, Chandraker A, Alan Brookhart M, Kramar R, Sunder-Plassmann G: A prospective study of anaemia and long-term outcomes in kidney transplant recipients. Nephrol Dial Transplant 21: 3559–3566, 2006 - PubMed
-
- Vanrenterghem Y, Ponticelli C, Morales JM, Abramowicz D, Baboolal K, Eklund B, Kliem V, Legendre C, Morais Sarmento AL, Vincenti F: Prevalence and management of anemia in renal transplant recipients: A European survey. Am J Transplant 3: 835–845, 2003 - PubMed
-
- Vanrenterghem Y: Anaemia after renal transplantation. Nephrol Dial Transplant 19[Suppl 5]: V54–V58, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

