Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing
- PMID: 24722454
- PMCID: PMC3983056
- DOI: 10.1371/journal.ppat.1004071
Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing
Abstract
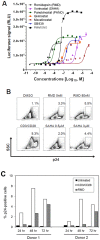
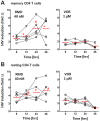
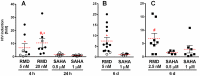
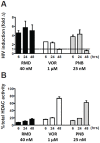
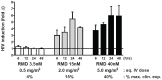
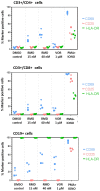
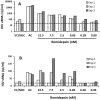
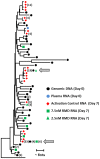
Persistent latent reservoir of replication-competent proviruses in memory CD4 T cells is a major obstacle to curing HIV infection. Pharmacological activation of HIV expression in latently infected cells is being explored as one of the strategies to deplete the latent HIV reservoir. In this study, we characterized the ability of romidepsin (RMD), a histone deacetylase inhibitor approved for the treatment of T-cell lymphomas, to activate the expression of latent HIV. In an in vitro T-cell model of HIV latency, RMD was the most potent inducer of HIV (EC50 = 4.5 nM) compared with vorinostat (VOR; EC50 = 3,950 nM) and other histone deacetylase (HDAC) inhibitors in clinical development including panobinostat (PNB; EC50 = 10 nM). The HIV induction potencies of RMD, VOR, and PNB paralleled their inhibitory activities against multiple human HDAC isoenzymes. In both resting and memory CD4 T cells isolated from HIV-infected patients on suppressive combination antiretroviral therapy (cART), a 4-hour exposure to 40 nM RMD induced a mean 6-fold increase in intracellular HIV RNA levels, whereas a 24-hour treatment with 1 µM VOR resulted in 2- to 3-fold increases. RMD-induced intracellular HIV RNA expression persisted for 48 hours and correlated with sustained inhibition of cell-associated HDAC activity. By comparison, the induction of HIV RNA by VOR and PNB was transient and diminished after 24 hours. RMD also increased levels of extracellular HIV RNA and virions from both memory and resting CD4 T-cell cultures. The activation of HIV expression was observed at RMD concentrations below the drug plasma levels achieved by doses used in patients treated for T-cell lymphomas. In conclusion, RMD induces HIV expression ex vivo at concentrations that can be achieved clinically, indicating that the drug may reactivate latent HIV in patients on suppressive cART.
Conflict of interest statement
DGW, VC, MB, TB, MG, JH, AI, JPM, GS, KMS, AT, HY, DS, RG, and TC are employees of Gilead Sciences, Inc. JM is a scientific advisor of Gilead Sciences, Inc. JL is a director of Quest Clinical Research that provided clinical specimens. EF, JS, MK, CAS, and DM have no competing interests. Part of the study was funded by Gilead Sciences, Inc. Employees of the company were involved in study design, data collection and analysis, and writing the manuscript. However, none of the therapeutics characterized in this study are products of Gilead Sciences, and studies were designed, executed, and objectively interpreted purely with a purpose to advance basic research in the field of HIV latency and cure. This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures
References
-
- DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, et al. (2012) Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 379: 2429–2438. - PubMed
-
- Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, et al. (2013) Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 381: 735–743. - PubMed
-
- Chun TW, Davey RT Jr, Ostrowski M, Shawn Justement J, Engel D, et al. (2000) Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med 6: 757–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

