A new mosaic pattern in glioma vascularization: exogenous endothelial progenitor cells integrating into the vessels containing tumor-derived endothelial cells
- PMID: 24722469
- PMCID: PMC4039108
- DOI: 10.18632/oncotarget.1885
A new mosaic pattern in glioma vascularization: exogenous endothelial progenitor cells integrating into the vessels containing tumor-derived endothelial cells
Abstract
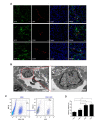
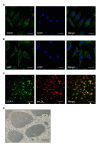
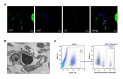
Emerging evidence suggests that glioma stem-like cells (GSCs) transdifferentiating into vascular endothelial cells (ECs) possibly contributes to tumor resistance to antiangiogenic therapy. Endothelial progenitor cells (EPCs), showing active migration and incorporation into neovasculature of glioma, may be a good vehicle for delivering genes to target GSCs transdifferentiation. Here, we found a new mosaic pattern that exogenous EPCs integrated into the vessels containing the tumor-derived ECs in C6 glioma rat model. Further, we evaluated the effect of these homing EPCs on C6 glioma cells transdifferentiation. The transdifferentiation frequency of C6 glioma cells and the expressions of key factors on GSCs transdifferentiation, i.e. HIF-1α, Notch1, and Flk1 in gliomas with or without EPCs transplantation showed no significant difference. Additionally, magnetic resonance imaging could track the migration and incorporation of EPCs into glioma in vivo, which was confirmed by Prussian blue staining. The number of magnetically labeled EPCs estimated from T2 maps correlated well with direct measurements of labeled cell counts by flow cytometry. Taken together, our findings may provide a rational base for the future application of EPCs as a therapeutic and imaging probe to overcome antiangiogenic resistance for glioma and monitor the efficacy of this treatment.
Figures




References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. - PubMed
-
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

