Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever
- PMID: 24722586
- PMCID: PMC3983105
- DOI: 10.1371/journal.pntd.0002790
Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever
Abstract
Background: Development of antiviral drugs that have broad-spectrum activity against a number of viral infections would be of significant benefit. Due to the evolution of resistance to currently licensed antiviral drugs, development of novel anti-influenza drugs is in progress, including Favipiravir (T-705), which is currently in human clinical trials. T-705 displays broad-spectrum in vitro activity against a number of viruses, including Rift Valley Fever virus (RVFV). RVF is an important neglected tropical disease that causes human, agricultural, and economic losses in endemic regions. RVF has the capacity to emerge in new locations and also presents a potential bioterrorism threat. In the current study, the in vivo efficacy of T-705 was evaluated in Wistar-Furth rats infected with the virulent ZH501 strain of RVFV by the aerosol route.
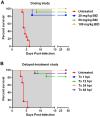
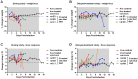
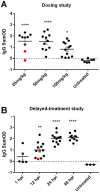
Methodology/principal findings: Wistar-Furth rats are highly susceptible to a rapidly lethal disease after parenteral or inhalational exposure to the pathogenic ZH501 strain of RVFV. In the current study, two experiments were performed: a dose-determination study and a delayed-treatment study. In both experiments, all untreated control rats succumbed to disease. Out of 72 total rats infected with RVFV and treated with T-705, only 6 succumbed to disease. The remaining 66 rats (92%) survived lethal infection with no significant weight loss or fever. The 6 treated rats that succumbed survived significantly longer before succumbing to encephalitic disease.
Conclusions/significance: Currently, there are no licensed antiviral drugs for treating RVF. Here, T-705 showed remarkable efficacy in a highly lethal rat model of Rift Valley Fever, even when given up to 48 hours post-infection. This is the first study to show protection of rats infected with the pathogenic ZH501 strain of RVFV. Our data suggest that T-705 has potential to be a broad-spectrum antiviral drug.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Samson M, Pizzorno A, Abed Y, Boivin G (2013) Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 98: 174–185. - PubMed
-
- FujiFilm Pharmaceuticals U.S.A Inc. (2011–2013) Pharmacokinetics of Favipiravir in Volunteers With Hepatic Impairment. In: ClinicalTrials.gov, editor. Bethesda, MD: National Library of Medicine.
-
- FujiFilm Pharmaceuticals U.S.A Inc. (2010–2012) Dose-Finding Study of Favipiravir in the Treatment of Uncomplicated Influenza. In: ClinicalTrials.gov, editor. US: National Library of Medicine.
-
- FujiFilm Pharmaceuticals U.S.A Inc., and Department of Defense. (2012–2013) T-705a Multicenter Study in Adults Subjects With Uncomplicated Influenza (FAVOR). In: ClinicalTrials.gov, editor. US: National Library of Medicine.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

