Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers
- PMID: 24722773
- PMCID: PMC3983045
- DOI: 10.1371/journal.ppat.1004045
Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers
Abstract
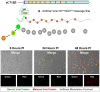
Coxsackievirus B3 (CVB3), a member of the picornavirus family and enterovirus genus, causes viral myocarditis, aseptic meningitis, and pancreatitis in humans. We genetically engineered a unique molecular marker, "fluorescent timer" protein, within our infectious CVB3 clone and isolated a high-titer recombinant viral stock (Timer-CVB3) following transfection in HeLa cells. "Fluorescent timer" protein undergoes slow conversion of fluorescence from green to red over time, and Timer-CVB3 can be utilized to track virus infection and dissemination in real time. Upon infection with Timer-CVB3, HeLa cells, neural progenitor and stem cells (NPSCs), and C2C12 myoblast cells slowly changed fluorescence from green to red over 72 hours as determined by fluorescence microscopy or flow cytometric analysis. The conversion of "fluorescent timer" protein in HeLa cells infected with Timer-CVB3 could be interrupted by fixation, suggesting that the fluorophore was stabilized by formaldehyde cross-linking reactions. Induction of a type I interferon response or ribavirin treatment reduced the progression of cell-to-cell virus spread in HeLa cells or NPSCs infected with Timer-CVB3. Time lapse photography of partially differentiated NPSCs infected with Timer-CVB3 revealed substantial intracellular membrane remodeling and the assembly of discrete virus replication organelles which changed fluorescence color in an asynchronous fashion within the cell. "Fluorescent timer" protein colocalized closely with viral 3A protein within virus replication organelles. Intriguingly, infection of partially differentiated NPSCs or C2C12 myoblast cells induced the release of abundant extracellular microvesicles (EMVs) containing matured "fluorescent timer" protein and infectious virus representing a novel route of virus dissemination. CVB3 virions were readily observed within purified EMVs by transmission electron microscopy, and infectious virus was identified within low-density isopycnic iodixanol gradient fractions consistent with membrane association. The preferential detection of the lipidated form of LC3 protein (LC3 II) in released EMVs harboring infectious virus suggests that the autophagy pathway plays a crucial role in microvesicle shedding and virus release, similar to a process previously described as autophagosome-mediated exit without lysis (AWOL) observed during poliovirus replication. Through the use of this novel recombinant virus which provides more dynamic information from static fluorescent images, we hope to gain a better understanding of CVB3 tropism, intracellular membrane reorganization, and virus-associated microvesicle dissemination within the host.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures













References
-
- Muir P, van Loon AM (1997) Enterovirus infections of the central nervous system. Intervirology 40: 153–166. - PubMed
-
- Sawyer MH (2002) Enterovirus infections: diagnosis and treatment. Semin Pediatr Infect Dis 13: 40–47. - PubMed
-
- Ornoy A, Tenenbaum A (2006) Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod Toxicol 21: 446–457. - PubMed
-
- Euscher E, Davis J, Holzman I, Nuovo GJ (2001) Coxsackie virus infection of the placenta associated with neurodevelopmental delays in the newborn. Obstet Gynecol 98: 1019–1026. - PubMed
-
- David P, Baleriaux D, Bank WO, Amrom D, De TD, et al. (1993) MRI of acute disseminated encephalomyelitis after coxsackie B infection. J Neuroradiol 20: 258–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

