Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates
- PMID: 24722905
- PMCID: PMC4024727
- DOI: 10.1152/ajpgi.00357.2013
Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates
Abstract
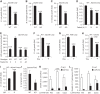
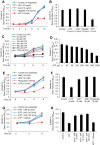
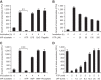
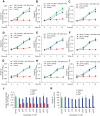
The intestinal microbiota plays a pivotal role in maintaining human health and well-being. Previously, we have shown that mice deficient in the brush-border enzyme intestinal alkaline phosphatase (IAP) suffer from dysbiosis and that oral IAP supplementation normalizes the gut flora. Here we aimed to decipher the molecular mechanism by which IAP promotes bacterial growth. We used an isolated mouse intestinal loop model to directly examine the effect of exogenous IAP on the growth of specific intestinal bacterial species. We studied the effects of various IAP targets on the growth of stool aerobic and anaerobic bacteria as well as on a few specific gut organisms. We determined the effects of ATP and other nucleotides on bacterial growth. Furthermore, we examined the effects of IAP on reversing the inhibitory effects of nucleotides on bacterial growth. We have confirmed that local IAP bioactivity creates a luminal environment that promotes the growth of a wide range of commensal organisms. IAP promotes the growth of stool aerobic and anaerobic bacteria and appears to exert its growth promoting effects by inactivating (dephosphorylating) luminal ATP and other luminal nucleotide triphosphates. We observed that compared with wild-type mice, IAP-knockout mice have more ATP in their luminal contents, and exogenous IAP can reverse the ATP-mediated inhibition of bacterial growth in the isolated intestinal loop. In conclusion, IAP appears to promote the growth of intestinal commensal bacteria by inhibiting the concentration of luminal nucleotide triphosphates.
Keywords: CpG DNA; dysbiosis; flagellin; gut flora; intestinal loop model; lipopolysaccharides; microbiotal homeostasis.
Copyright © 2014 the American Physiological Society.
Figures
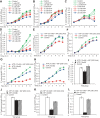
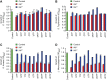
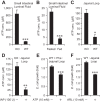
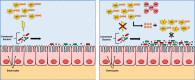
Comment in
-
Luminal ATP: the missing link between intestinal alkaline phosphatase, the gut microbiota, and inflammation?Am J Physiol Gastrointest Liver Physiol. 2014 May 15;306(10):G824-5. doi: 10.1152/ajpgi.00435.2013. Epub 2014 Mar 27. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24674777 No abstract available.
Similar articles
-
Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota.Gut. 2010 Nov;59(11):1476-84. doi: 10.1136/gut.2010.211706. Gut. 2010. PMID: 20947883
-
Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase.Am J Physiol Gastrointest Liver Physiol. 2010 Aug;299(2):G467-75. doi: 10.1152/ajpgi.00364.2009. Epub 2010 May 20. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20489044 Free PMC article.
-
Loss of Intestinal Alkaline Phosphatase Leads to Distinct Chronic Changes in Bone Phenotype.J Surg Res. 2018 Dec;232:325-331. doi: 10.1016/j.jss.2018.06.061. Epub 2018 Jul 18. J Surg Res. 2018. PMID: 30463736
-
Intestinal alkaline phosphatase: novel functions and protective effects.Nutr Rev. 2014 Feb;72(2):82-94. doi: 10.1111/nure.12082. Epub 2013 Dec 9. Nutr Rev. 2014. PMID: 24506153 Review.
-
The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract.Mediators Inflamm. 2017;2017:9074601. doi: 10.1155/2017/9074601. Epub 2017 Feb 21. Mediators Inflamm. 2017. PMID: 28316376 Free PMC article. Review.
Cited by
-
Alkaline Phosphatase and Hypophosphatasia.Calcif Tissue Int. 2016 Apr;98(4):398-416. doi: 10.1007/s00223-015-0079-1. Epub 2015 Nov 21. Calcif Tissue Int. 2016. PMID: 26590809 Free PMC article. Review.
-
Investigation of the relationship between atopic dermatitis of dogs and intestinal epithelial damage.Vet Med Sci. 2024 May;10(3):e1453. doi: 10.1002/vms3.1453. Vet Med Sci. 2024. PMID: 38648253 Free PMC article.
-
The gut-liver axis in sepsis: interaction mechanisms and therapeutic potential.Crit Care. 2022 Jul 13;26(1):213. doi: 10.1186/s13054-022-04090-1. Crit Care. 2022. PMID: 35831877 Free PMC article. Review.
-
Intestinal alkaline phosphatase deficiency leads to lipopolysaccharide desensitization and faster weight gain.Infect Immun. 2015 Jan;83(1):247-58. doi: 10.1128/IAI.02520-14. Epub 2014 Oct 27. Infect Immun. 2015. PMID: 25348635 Free PMC article.
-
Microbiota-host interplay at the gut epithelial level, health and nutrition.J Anim Sci Biotechnol. 2016 Nov 8;7:66. doi: 10.1186/s40104-016-0123-7. eCollection 2016. J Anim Sci Biotechnol. 2016. PMID: 27833747 Free PMC article. Review.
References
-
- Alam SN, Yammine H, Moaven O, Ahmed R, Moss AK, Biswas B, Muhammad N, Biswas R, Raychowdhury A, Kaliannan K, Ghosh S, Ray M, Hamarneh SR, Barua S, Malo NS, Bhan AK, Malo MS, Hodin RA. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann Surg 259: 715–722, 2014 - PMC - PubMed
-
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria TH17 cell differentiation. Nature 455: 808–812, 2008 - PubMed
-
- Balakrishnan M, Floch MH. Prebiotics, probiotics and digestive health. Curr Opin Clin Nutr Metab Care 15: 580–585, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

