Differential regulation of the two-component regulatory system senX3-regX3 in Mycobacterium tuberculosis
- PMID: 24722908
- PMCID: PMC4039243
- DOI: 10.1099/mic.0.077180-0
Differential regulation of the two-component regulatory system senX3-regX3 in Mycobacterium tuberculosis
Abstract
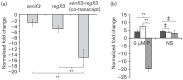
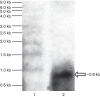
The highly successful pathogen Mycobacterium tuberculosis (Mtb) has evolved strategies to adapt to various stress conditions, thus promoting survival within the infected host. The two-component regulatory system (2CRS) senX3-regX3, which has been implicated in the Mtb response to inorganic phosphate depletion, is believed to behave as an auto-regulatory bicistronic operon. Unlike other 2CRS, Mtb senX3-regX3 features an intergenic region (IR) containing several mycobacterium interspersed repetitive units (MIRU) of unknown function. In this study, we used a lacZ reporter system to study the promoter activity of the 5' untranslated region of senX3, and that of various numbers of MIRUs in the senX3-regX3 IR, during axenic Mtb growth in nutrient-rich broth, and upon exposure to growth-restricting conditions. Activity of the senX3 promoter was induced during phosphate depletion and nutrient starvation, and IR promoter activity under these conditions was directly proportional to the number of MIRUs present. Quantitative reverse transcriptase (qRT)-PCR analysis of exponentially growing Mtb revealed monocistronic transcription of senX3 and regX3, and, to a lesser degree, bicistronic transcription of the operon. In addition, we observed primarily monocistronic upregulation of regX3 during phosphate depletion of Mtb, which was confirmed by Northern analysis in wild-type Mtb and by RT-PCR in a senX3-disrupted mutant, while upregulation of regX3 in nutrient-starved Mtb was chiefly bicistronic. Our findings of differential regulation of senX3-regX3 highlight the potential regulatory role of MIRUs in the Mtb genome and provide insight into the regulatory mechanisms underlying Mtb adaptation to physiologically relevant conditions.
© 2014 The Authors.
Figures



References
-
- Baek J. H., Lee S. Y. (2007). Transcriptome analysis of phosphate starvation response in Escherichia coli. J Microbiol Biotechnol 17, 244–252. - PubMed
-
- Barletta R. G., Kim D. D., Snapper S. B., Bloom B. R., Jacobs W. R., Jr (1992). Identification of expression signals of the mycobacteriophages Bxb1, L1 and TM4 using the Escherichia-Mycobacterium shuttle plasmids pYUB75 and pYUB76 designed to create translational fusions to the lacZ gene. J Gen Microbiol 138, 23–30. 10.1099/00221287-138-1-23 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

