Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system
- PMID: 24722990
- PMCID: PMC4140916
- DOI: 10.1074/jbc.M113.533653
Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system
Abstract
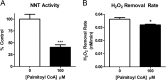
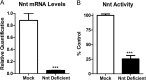
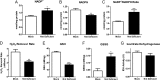
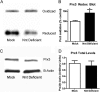
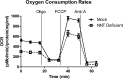
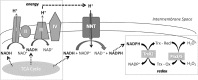
Mitochondrial reactive oxygen species are implicated in the etiology of multiple neurodegenerative diseases, including Parkinson disease. Mitochondria are known to be net producers of ROS, but recently we have shown that brain mitochondria can consume mitochondrial hydrogen peroxide (H2O2) in a respiration-dependent manner predominantly by the thioredoxin/peroxiredoxin system. Here, we sought to determine the mechanism linking mitochondrial respiration with H2O2 catabolism in brain mitochondria and dopaminergic cells. We hypothesized that nicotinamide nucleotide transhydrogenase (Nnt), which utilizes the proton gradient to generate NADPH from NADH and NADP(+), provides the link between mitochondrial respiration and H2O2 detoxification through the thioredoxin/peroxiredoxin system. Pharmacological inhibition of Nnt in isolated brain mitochondria significantly decreased their ability to consume H2O2 in the presence, but not absence, of respiration substrates. Nnt inhibition in liver mitochondria, which do not require substrates to detoxify H2O2, had no effect. Pharmacological inhibition or lentiviral knockdown of Nnt in N27 dopaminergic cells (a) decreased H2O2 catabolism, (b) decreased NADPH and increased NADP(+) levels, and (c) decreased basal, spare, and maximal mitochondrial oxygen consumption rates. Nnt-deficient cells possessed higher levels of oxidized mitochondrial Prx, which rendered them more susceptible to steady-state increases in H2O2 and cell death following exposure to subtoxic levels of paraquat. These data implicate Nnt as the critical link between the metabolic and H2O2 antioxidant function in brain mitochondria and suggests Nnt as a potential therapeutic target to improve the redox balance in conditions of oxidative stress associated with neurodegenerative diseases.
Keywords: Mitochondria; Nicotinamide Nucleotide Transhydrogenase; Oxidative Stress; Parkinson Disease; Reactive Oxygen Species (ROS); Thioredoxin Reductase.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Thioredoxin reductase deficiency potentiates oxidative stress, mitochondrial dysfunction and cell death in dopaminergic cells.PLoS One. 2012;7(11):e50683. doi: 10.1371/journal.pone.0050683. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226354 Free PMC article.
-
Nicotinamide nucleotide transhydrogenase is required for brain mitochondrial redox balance under hampered energy substrate metabolism and high-fat diet.J Neurochem. 2018 Dec;147(5):663-677. doi: 10.1111/jnc.14602. Epub 2018 Nov 26. J Neurochem. 2018. PMID: 30281804
-
Brain mitochondria from DJ-1 knockout mice show increased respiration-dependent hydrogen peroxide consumption.Redox Biol. 2014 Apr 24;2:667-72. doi: 10.1016/j.redox.2014.04.010. eCollection 2014. Redox Biol. 2014. PMID: 24936441 Free PMC article.
-
Mitochondrial NAD(P)+ Transhydrogenase: From Molecular Features to Physiology and Disease.Antioxid Redox Signal. 2022 May;36(13-15):864-884. doi: 10.1089/ars.2021.0111. Epub 2021 Aug 5. Antioxid Redox Signal. 2022. PMID: 34155914 Review.
-
Mitochondrial Nicotinamide Nucleotide Transhydrogenase: Role in Energy Metabolism, Redox Homeostasis, and Cancer.Antioxid Redox Signal. 2024 Nov;41(13-15):927-956. doi: 10.1089/ars.2024.0694. Antioxid Redox Signal. 2024. PMID: 39585234 Review.
Cited by
-
Vitamin E and vitamin C do not reduce insulin sensitivity but inhibit mitochondrial protein expression in exercising obese rats.Appl Physiol Nutr Metab. 2015 Apr;40(4):343-52. doi: 10.1139/apnm-2014-0302. Epub 2014 Dec 9. Appl Physiol Nutr Metab. 2015. PMID: 25761734 Free PMC article.
-
Factors Influencing Mitochondrial Function as a Key Mediator of Glucose-Induced Insulin Release: Highlighting Nicotinamide Nucleotide Transhydrogenase.Int J Mol Cell Med. 2020 Spring;9(2):107-122. doi: 10.22088/IJMCM.BUMS.9.2.107. Epub 2020 Aug 10. Int J Mol Cell Med. 2020. PMID: 32934948 Free PMC article. Review.
-
An epigenome-wide analysis of socioeconomic position and tumor DNA methylation in breast cancer patients.Clin Epigenetics. 2023 Apr 26;15(1):68. doi: 10.1186/s13148-023-01470-4. Clin Epigenetics. 2023. PMID: 37101222 Free PMC article.
-
Glia Maturation Factor Dependent Inhibition of Mitochondrial PGC-1α Triggers Oxidative Stress-Mediated Apoptosis in N27 Rat Dopaminergic Neuronal Cells.Mol Neurobiol. 2018 Sep;55(9):7132-7152. doi: 10.1007/s12035-018-0882-6. Epub 2018 Jan 30. Mol Neurobiol. 2018. PMID: 29383690 Free PMC article.
-
Upregulation of mitochondrial NAD+ levels impairs the clonogenicity of SSEA1+ glioblastoma tumor-initiating cells.Exp Mol Med. 2017 Jun 9;49(6):e344. doi: 10.1038/emm.2017.74. Exp Mol Med. 2017. PMID: 28604662 Free PMC article.
References
-
- Halliwell B., Gutteridge J. (2007) Free Radicals in Biology and Medicine, 4th Ed., pp. 25–110, Oxford University Press, New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

