Interneurons from embryonic development to cell-based therapy
- PMID: 24723614
- PMCID: PMC4056344
- DOI: 10.1126/science.1240622
Interneurons from embryonic development to cell-based therapy
Abstract
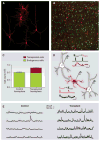
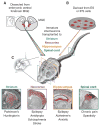
Many neurologic and psychiatric disorders are marked by imbalances between neural excitation and inhibition. In the cerebral cortex, inhibition is mediated largely by GABAergic (γ-aminobutyric acid-secreting) interneurons, a cell type that originates in the embryonic ventral telencephalon and populates the cortex through long-distance tangential migration. Remarkably, when transplanted from embryos or in vitro culture preparations, immature interneurons disperse and integrate into host brain circuits, both in the cerebral cortex and in other regions of the central nervous system. These features make interneuron transplantation a powerful tool for the study of neurodevelopmental processes such as cell specification, cell death, and cortical plasticity. Moreover, interneuron transplantation provides a novel strategy for modifying neural circuits in rodent models of epilepsy, Parkinson's disease, mood disorders, and chronic pain.
Figures

References
Publication types
MeSH terms
Grants and funding
- MH049428/MH/NIMH NIH HHS/United States
- R01 NS028478/NS/NINDS NIH HHS/United States
- NS28478/NS/NINDS NIH HHS/United States
- R01 NS014627/NS/NINDS NIH HHS/United States
- NS78326/NS/NINDS NIH HHS/United States
- R01-EY02874/EY/NEI NIH HHS/United States
- R37 MH049428/MH/NIMH NIH HHS/United States
- R37 NS028478/NS/NINDS NIH HHS/United States
- HD032116/HD/NICHD NIH HHS/United States
- NS14627/NS/NINDS NIH HHS/United States
- R37 NS014627/NS/NINDS NIH HHS/United States
- R01 EY002874/EY/NEI NIH HHS/United States
- R37 HD032116/HD/NICHD NIH HHS/United States
- T32 GM008568/GM/NIGMS NIH HHS/United States
- R01 NS078326/NS/NINDS NIH HHS/United States
- R01 MH049428/MH/NIMH NIH HHS/United States
- R01 HD032116/HD/NICHD NIH HHS/United States
- P30 EY002162/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

