TPL2 kinase regulates the inflammatory milieu of the myeloma niche
- PMID: 24723682
- PMCID: PMC4046426
- DOI: 10.1182/blood-2014-02-554071
TPL2 kinase regulates the inflammatory milieu of the myeloma niche
Abstract
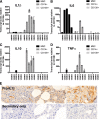
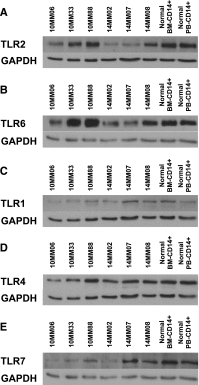
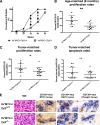
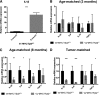
Targeted modulation of microenvironmental regulatory pathways may be essential to control myeloma and other genetically/clonally heterogeneous cancers. Here we report that human myeloma-associated monocytes/macrophages (MAM), but not myeloma plasma cells, constitute the predominant source of interleukin-1β (IL-1β), IL-10, and tumor necrosis factor-α at diagnosis, whereas IL-6 originates from stromal cells and macrophages. To dissect MAM activation/cytokine pathways, we analyzed Toll-like receptor (TLR) expression in human myeloma CD14(+) cells. We observed coregulation of TLR2 and TLR6 expression correlating with local processing of versican, a proteoglycan TLR2/6 agonist linked to carcinoma progression. Versican has not been mechanistically implicated in myeloma pathogenesis. We hypothesized that the most readily accessible target in the versican-TLR2/6 pathway would be the mitogen-activated protein 3 (MAP3) kinase, TPL2 (Cot/MAP3K8). Ablation of Tpl2 in the genetically engineered in vivo myeloma model, Vκ*MYC, led to prolonged disease latency associated with plasma cell growth defect. Tpl2 loss abrogated the "inflammatory switch" in MAM within nascent myeloma lesions and licensed macrophage repolarization in established tumors. MYC activation/expression in plasma cells was independent of Tpl2 activity. Pharmacologic TPL2 inhibition in human monocytes led to dose-dependent attenuation of IL-1β induction/secretion in response to TLR2 stimulation. Our results highlight a TLR2/6-dependent TPL2 pathway as novel therapeutic target acting nonautonomously through macrophages to control myeloma progression.
© 2014 by The American Society of Hematology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

