Carbonate hydroxyapatite and silicon-substituted carbonate hydroxyapatite: synthesis, mechanical properties, and solubility evaluations
- PMID: 24723840
- PMCID: PMC3958659
- DOI: 10.1155/2014/969876
Carbonate hydroxyapatite and silicon-substituted carbonate hydroxyapatite: synthesis, mechanical properties, and solubility evaluations
Abstract
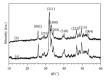
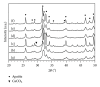
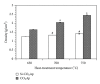
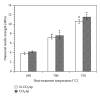
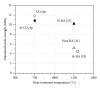
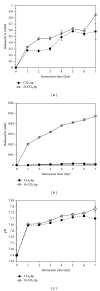
The present study investigates the chemical composition, solubility, and physical and mechanical properties of carbonate hydroxyapatite (CO3Ap) and silicon-substituted carbonate hydroxyapatite (Si-CO3Ap) which have been prepared by a simple precipitation method. X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), X-ray fluorescence (XRF) spectroscopy, and inductively coupled plasma (ICP) techniques were used to characterize the formation of CO3Ap and Si-CO3Ap. The results revealed that the silicate (SiO4(4-)) and carbonate (CO3(2-)) ions competed to occupy the phosphate (PO4(3-)) site and also entered simultaneously into the hydroxyapatite structure. The Si-substituted CO3Ap reduced the powder crystallinity and promoted ion release which resulted in a better solubility compared to that of Si-free CO3Ap. The mean particle size of Si-CO3Ap was much finer than that of CO3Ap. At 750°C heat-treatment temperature, the diametral tensile strengths (DTS) of Si-CO3Ap and CO3Ap were about 10.8 ± 0.3 and 11.8 ± 0.4 MPa, respectively.
Figures

References
-
- Dorozhkin SV. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomaterialia. 2010;6(3):715–734. - PubMed
-
- Wagoner Johnson AJ, Herschler BA. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomaterialia. 2011;7(1):16–30. - PubMed
-
- Gomes S, Nedelec J-M, Jallot E, Sheptyakov D, Renaudin G. Silicon location in silicate-substituted calcium phosphate ceramics determined by neutron diffraction. Crystal Growth and Design. 2011;11(9):4017–4026.
-
- Kolmas J, Jaklewicz A, Zima A, et al. Incorporation of carbonate and magnesium ions into synthetic hydroxyapatite: the effect on physicochemical properties. Journal of Molecular Structure. 2011;987(1–3):40–50.
-
- Lombardi M, Palmero P, Haberko K, Pyda W, Montanaro L. Processing of a natural hydroxyapatite powder: from powder optimization to porous bodies development. Journal of the European Ceramic Society. 2011;31(14):2513–2518.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

