Modulatory mechanisms and multiple functions of somatodendritic A-type K (+) channel auxiliary subunits
- PMID: 24723849
- PMCID: PMC3973911
- DOI: 10.3389/fncel.2014.00082
Modulatory mechanisms and multiple functions of somatodendritic A-type K (+) channel auxiliary subunits
Abstract
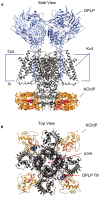
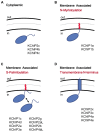
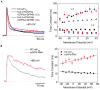
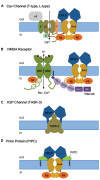
Auxiliary subunits are non-conducting, modulatory components of the multi-protein ion channel complexes that underlie normal neuronal signaling. They interact with the pore-forming α-subunits to modulate surface distribution, ion conductance, and channel gating properties. For the somatodendritic subthreshold A-type potassium (ISA) channel based on Kv4 α-subunits, two types of auxiliary subunits have been extensively studied: Kv channel-interacting proteins (KChIPs) and dipeptidyl peptidase-like proteins (DPLPs). KChIPs are cytoplasmic calcium-binding proteins that interact with intracellular portions of the Kv4 subunits, whereas DPLPs are type II transmembrane proteins that associate with the Kv4 channel core. Both KChIPs and DPLPs genes contain multiple start sites that are used by various neuronal populations to drive the differential expression of functionally distinct N-terminal variants. In turn, these N-terminal variants generate tremendous functional diversity across the nervous system. Here, we focus our review on (1) the molecular mechanism underlying the unique properties of different N-terminal variants, (2) the shaping of native ISA properties by the concerted actions of KChIPs and DPLP variants, and (3) the surprising ways that KChIPs and DPLPs coordinate the activity of multiple channels to fine-tune neuronal excitability. Unlocking the unique contributions of different auxiliary subunit N-terminal variants may provide an important opportunity to develop novel targeted therapeutics to treat numerous neurological disorders.
Keywords: Kv channel-interacting protein; N-terminal variant; auxiliary subunit; dipeptidyl peptidase-like protein; excitability; modulatory mechanism; potassium channel; somatodendritic A-type current.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

