Irrelevant stimulus processing in ADHD: catecholamine dynamics and attentional networks
- PMID: 24723897
- PMCID: PMC3972460
- DOI: 10.3389/fpsyg.2014.00183
Irrelevant stimulus processing in ADHD: catecholamine dynamics and attentional networks
Abstract
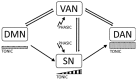
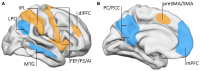
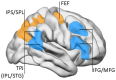
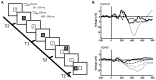
A cardinal symptom of attention deficit and hyperactivity disorder (ADHD) is a general distractibility where children and adults shift their attentional focus to stimuli that are irrelevant to the ongoing behavior. This has been attributed to a deficit in dopaminergic signaling in cortico-striatal networks that regulate goal-directed behavior. Furthermore, recent imaging evidence points to an impairment of large scale, antagonistic brain networks that normally contribute to attentional engagement and disengagement, such as the task-positive networks and the default mode network (DMN). Related networks are the ventral attentional network (VAN) involved in attentional shifting, and the salience network (SN) related to task expectancy. Here we discuss the tonic-phasic dynamics of catecholaminergic signaling in the brain, and attempt to provide a link between this and the activities of the large-scale cortical networks that regulate behavior. More specifically, we propose that a disbalance of tonic catecholamine levels during task performance produces an emphasis of phasic signaling and increased excitability of the VAN, yielding distractibility symptoms. Likewise, immaturity of the SN may relate to abnormal tonic signaling and an incapacity to build up a proper executive system during task performance. We discuss different lines of evidence including pharmacology, brain imaging and electrophysiology, that are consistent with our proposal. Finally, restoring the pharmacodynamics of catecholaminergic signaling seems crucial to alleviate ADHD symptoms; however, the possibility is open to explore cognitive rehabilitation strategies to top-down modulate network dynamics compensating the pharmacological deficits.
Keywords: CNV; P300; attention; fMRI; ventral attentional network.
Figures
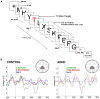
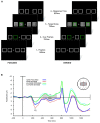
References
-
- Aboitiz F. (2009a). “Dynamics of a neuromodulator: I. The role of dopaminergic signaling in goal-directed behavior,” in From Attention to Goal-Directed Behavior. Neurodynamical, Methodological and Clinical Trends eds Aboitiz F., Cosmelli D. (Berlin: Springer; ) 187–204 10.1007/978-3-540-70573-4_10 - DOI
-
- Aboitiz F. (2009b). “Dynamics of a neuromodulator: II. Dopaminergic balance and cognition,” in From Attention to Goal-Directed Behavior. Neurodynamical, Methodological and Clinical Trends eds Aboitiz F., Cosmelli D. (Berlin: Springer; ) 205–227 10.1007/978-3-540-70573-4_11 - DOI
-
- Aboitiz F., Carrasco X., Castellanos F. X. (2010). “Attention-deficit and disruptive behavior disorders,” in Encyclopaedia of Psychopharmacology ed. Stolerman I. (New York: Springer)
-
- Aboitiz F., Castellanos F. X. (2011). “ADHD, catecholamines and the default mode of brain function. A reassessment of the dopaminergic hypothesis of ADHD,” in Attention Deficit Hyperactivity Disorder vol. 2 eds Evans S., Hoza B. (Kingston, NJ: Civic Research Institute; ) 2-1–2-13
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

