Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management
- PMID: 24723942
- PMCID: PMC3958662
- DOI: 10.1155/2014/734249
Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management
Abstract
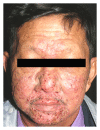
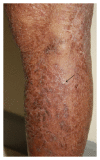
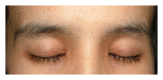
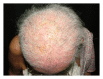
Epidermal growth factor inhibitors (EGFRI), the first targeted cancer therapy, are currently an essential treatment for many advance-stage epithelial cancers. These agents have the superior ability to target cancers cells and better safety profile compared to conventional chemotherapies. However, cutaneous adverse events are common due to the interference of epidermal growth factor receptor (EGFR) signaling in the skin. Cutaneous toxicities lead to poor compliance, drug cessation, and psychosocial discomfort. This paper summarizes the current knowledge concerning the presentation and management of skin toxicity from EGFRI. The common dermatologic adverse events are papulopustules and xerosis. Less common findings are paronychia, regulatory abnormalities of hair growth, maculopapular rash, mucositis, and postinflammatory hyperpigmentation. Radiation enhances EGFRI rash due to synergistic toxicity. There is a positive correlation between the occurrence and severity of cutaneous adverse effects and tumor response. To date, prophylactic systemic tetracycline and tetracycline class antibiotics have proven to be the most effective treatment regime.
Figures
References
-
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. The New England Journal of Medicine. 2008;358(11):1160–1174. - PubMed
-
- Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. Journal of Clinical Oncology. 2007;25(26):4057–4065. - PubMed
-
- Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an eastern cooperative oncology group study. Journal of Clinical Oncology. 2005;23(34):8646–8654. - PubMed
-
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan- refractory metastatic colorectal cancer. The New England Journal of Medicine. 2004;351(4):337–345. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

