Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics
- PMID: 24723986
- PMCID: PMC3982135
- DOI: 10.7150/thno.8698
Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics
Abstract
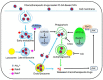
Nanotheranostics is to apply and further develop nanomedicine strategies for advanced theranostics. This review summarizes the various nanocarriers developed so far in the literature for nanotheranostics, which include polymer conjugations, dendrimers, micelles, liposomes, metal and inorganic nanoparticles, carbon nanotubes, and nanoparticles of biodegradable polymers for sustained, controlled and targeted co-delivery of diagnostic and therapeutic agents for better theranostic effects with fewer side effects. The theranostic nanomedicine can achieve systemic circulation, evade host defenses and deliver the drug and diagnostic agents at the targeted site to diagnose and treat the disease at cellular and molecular level. The therapeutic and diagnostic agents are formulated in nanomedicine as a single theranostic platform, which can then be further conjugated to biological ligand for targeting. Nanotheranostics can also promote stimuli-responsive release, synergetic and combinatory therapy, siRNA co-delivery, multimodality therapies, oral delivery, delivery across the blood-brain barrier as well as escape from intracellular autophagy. The fruition of nanotheranostics will be able to provide personalized therapy with bright prognosis, which makes even the fatal diseases curable or at least treatable at the earliest stage.
Keywords: Cancer nanotechnology; Drug targeting; Molecular biomaterials; Molecular imaging; Oral chemotherapy; Pharmaceutical nanotechnology..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
The upcoming field of theranostic nanomedicine: an overview.J Biomed Nanotechnol. 2012 Dec;8(6):859-82. doi: 10.1166/jbn.2012.1459. J Biomed Nanotechnol. 2012. PMID: 23029995 Review.
-
Nanotheranostics: advanced nanomedicine for the integration of diagnosis and therapy.Nanomedicine (Lond). 2014 Jul;9(9):1277-80. doi: 10.2217/nnm.14.83. Nanomedicine (Lond). 2014. PMID: 25204816 No abstract available.
-
Voyage of theranostic liposomes for imaging and therapy.J Cosmet Laser Ther. 2017 Aug;19(4):245-249. doi: 10.1080/14764172.2017.1279331. Epub 2017 Jan 31. J Cosmet Laser Ther. 2017. PMID: 28135901 Review.
-
Theranostic nanomedicine.Acc Chem Res. 2011 Oct 18;44(10):1029-38. doi: 10.1021/ar200019c. Epub 2011 May 5. Acc Chem Res. 2011. PMID: 21545096 Review.
-
Nanotheranostics for personalized medicine.Adv Drug Deliv Rev. 2012 Oct;64(13):1394-416. doi: 10.1016/j.addr.2012.06.006. Epub 2012 Jun 21. Adv Drug Deliv Rev. 2012. PMID: 22728642 Review.
Cited by
-
From Diagnosis to Treatment: Clinical Applications of Nanotechnology in Thoracic Surgery.Thorac Surg Clin. 2016 May;26(2):215-28. doi: 10.1016/j.thorsurg.2015.12.009. Thorac Surg Clin. 2016. PMID: 27112260 Free PMC article. Review.
-
Berberine mediated fluorescent gold nanoclusters in biomimetic erythrocyte ghosts as a nanocarrier for enhanced photodynamic treatment.RSC Adv. 2024 Jan 19;14(5):3321-3334. doi: 10.1039/d3ra08299g. eCollection 2024 Jan 17. RSC Adv. 2024. PMID: 38249664 Free PMC article.
-
Near-infrared photodynamic and photothermal co-therapy based on organic small molecular dyes.J Nanobiotechnology. 2023 Sep 27;21(1):348. doi: 10.1186/s12951-023-02111-x. J Nanobiotechnology. 2023. PMID: 37759287 Free PMC article. Review.
-
Combining Nanocarrier-Assisted Delivery of Molecules and Radiotherapy.Pharmaceutics. 2022 Jan 3;14(1):105. doi: 10.3390/pharmaceutics14010105. Pharmaceutics. 2022. PMID: 35057001 Free PMC article. Review.
-
SREBP1 siRNA enhance the docetaxel effect based on a bone-cancer dual-targeting biomimetic nanosystem against bone metastatic castration-resistant prostate cancer.Theranostics. 2020 Jan 1;10(4):1619-1632. doi: 10.7150/thno.40489. eCollection 2020. Theranostics. 2020. PMID: 32042326 Free PMC article.
References
-
- Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine (Lond) 2008;3(2):137–140. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

