Novel aspects of glucocorticoid actions
- PMID: 24724595
- PMCID: PMC4161987
- DOI: 10.1111/jne.12157
Novel aspects of glucocorticoid actions
Abstract
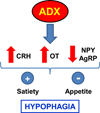
Normal hypothalamic-pituitary-adrenal (HPA) axis activity leading to the rhythmic and episodic release of adrenal glucocorticoids (GCs) is essential for body homeostasis and survival during stress. Acting through specific intracellular receptors in the brain and periphery, GCs regulate behaviour, as well as metabolic, cardiovascular, immune and neuroendocrine activities. By contrast to chronic elevated levels, circadian and acute stress-induced increases in GCs are necessary for hippocampal neuronal survival and memory acquisition and consolidation, as a result of the inhibition of apoptosis, the facilitation of glutamatergic neurotransmission and the formation of excitatory synapses, and the induction of immediate early genes and dendritic spine formation. In addition to metabolic actions leading to increased energy availability, GCs have profound effects on feeding behaviour, mainly via the modulation of orexigenic and anorixegenic neuropeptides. Evidence is also emerging that, in addition to the recognised immune suppressive actions of GCs by counteracting adrenergic pro-inflammatory actions, circadian elevations have priming effects in the immune system, potentiating acute defensive responses. In addition, negative-feedback by GCs involves multiple mechanisms leading to limited HPA axis activation and prevention of the deleterious effects of excessive GC production. Adequate GC secretion to meet body demands is tightly regulated by a complex neural circuitry controlling hypothalamic corticotrophin-releasing hormone (CRH) and vasopressin secretion, which are the main regulators of pituitary adrenocorticotrophic hormone (ACTH). Rapid feedback mechanisms, likely involving nongenomic actions of GCs, mediate the immediate inhibition of hypothalamic CRH and ACTH secretion, whereas intermediate and delayed mechanisms mediated by genomic actions involve the modulation of limbic circuitry and peripheral metabolic messengers. Consistent with their key adaptive roles, HPA axis components are evolutionarily conserved, being present in the earliest vertebrates. An understanding of these basic mechanisms may lead to novel approaches for the development of diagnostic and therapeutic tools for disorders related to stress and alterations of GC secretion.
Keywords: HPA axis; feedback; food intake; glucocorticoids; immune system; neuroplasticity.
© 2014 British Society for Neuroendocrinology.
Figures




References
-
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16(2):115–130. - PubMed
-
- Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583(2–3):255–262. - PubMed
-
- Joëls M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43(1):1–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

