Improving industrial yeast strains: exploiting natural and artificial diversity
- PMID: 24724938
- PMCID: PMC4293462
- DOI: 10.1111/1574-6976.12073
Improving industrial yeast strains: exploiting natural and artificial diversity
Abstract
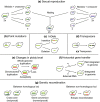
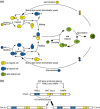
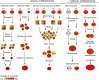
Yeasts have been used for thousands of years to make fermented foods and beverages, such as beer, wine, sake, and bread. However, the choice for a particular yeast strain or species for a specific industrial application is often based on historical, rather than scientific grounds. Moreover, new biotechnological yeast applications, such as the production of second-generation biofuels, confront yeast with environments and challenges that differ from those encountered in traditional food fermentations. Together, this implies that there are interesting opportunities to isolate or generate yeast variants that perform better than the currently used strains. Here, we discuss the different strategies of strain selection and improvement available for both conventional and nonconventional yeasts. Exploiting the existing natural diversity and using techniques such as mutagenesis, protoplast fusion, breeding, genome shuffling and directed evolution to generate artificial diversity, or the use of genetic modification strategies to alter traits in a more targeted way, have led to the selection of superior industrial yeasts. Furthermore, recent technological advances allowed the development of high-throughput techniques, such as 'global transcription machinery engineering' (gTME), to induce genetic variation, providing a new source of yeast genetic diversity.
Keywords: GMO; Saccharomyces cerevisiae; evolutionary engineering; genetic engineering; metabolic engineering; non-Saccharomyces.
© 2014 The Authors. FEMS Microbiology Reviews published by John Wiley & Sons Ltd on behalf of Federation of European Microbiological Societies.
Figures


References
-
- Aarnio T, Suikho M. Kauppinen V. Isolation of acetic acid tolerant baker's yeast variants in a turbidostat. Appl Biochem Biotechnol. 1991;27:55–63.
-
- Agbogbo FK. Coward-Kelly G. Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol Lett. 2008;30:1515–1524. - PubMed
-
- Akada R. Genetically modified industrial yeast ready for application. J Biosci Bioeng. 2002;94:536–544. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

