The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation
- PMID: 24725321
- PMCID: PMC3986807
- DOI: 10.1016/j.jtos.2013.10.007
The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation
Abstract
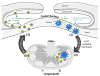
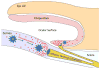
Dendritic cells (DCs) are highly potent stimulators of the immune system, and their contribution as such to the pathogenesis of corneal and ocular surface inflammatory disease has been well established. These vigorous antigen-presenting cells are reliant upon their effective migration from peripheral tissues (e.g., those of the ocular surface) to the lymphoid organs, where immune responses are triggered and can then cause disease. The chemokine receptor CCR7 expressed on DCs has emerged as the master mediator of this highly complex migratory process, and thus it is important in causing corneal and ocular surface inflammation. Furthermore, CCR7 has received considerable attention as a potential therapeutic target, as topically instilled antagonists of this receptor are quite effective therapeutically in a mouse model of ocular allergy. These findings and more are reviewed in the current article. In addition, the understanding regarding CCR7 function in mice and humans, and the biology of DCs that populate the ocular surface are also detailed herein. The involvement of DCs and their expression of CCR7 in corneal and ocular surface diseases such as in ocular allergy, dry eye disease, immune rejection and more, are also reviewed here.
Keywords: CCR7; CD103; T cells; allergic conjunctivitis; conjunctivitis; dendritic cells; dry eye disease; keratitis; ocular allergy; ocular surface.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure/Conflict of Interest: Author is inventor on patent application.
Figures







References
-
- Schaumburg CS, Siemasko KF, De Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

